|
The beta-adrenergic receptor may be
considered the prototype for GPCRs, but in fact there are six different
classes of receptors in the superfamily of GPCRs. The classes have been
constructed on the basis of sequence homology and structure. For the
neurotransmitters and neuropeptides, only the first three classes, termed
classes A, B and C, are important.
The
classes of proteins in the 7 transmembrane (7TM) GPCR superfamily are also often referred to by the name of a prominent member of
the class. Thus, we have the rhodopsin-like family, the calcitonin
receptor-like family or the metabotropic glutamate receptor family. The
class A receptors include receptors for biogenic amines such as
adrenaline, noradrenaline and dopamine. Some of the neuropeptide
receptors also come from this family. This class of receptors is
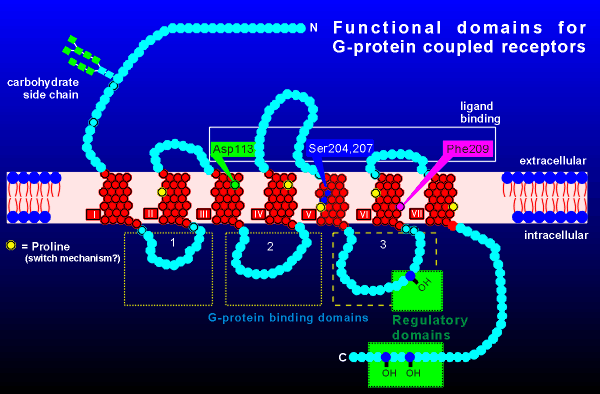
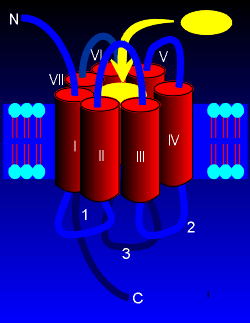
characterized by being heavily glycosylated at the N-terminal and
possessing a palmitolyation in the intracellular C-terminal region.
Palmitoylation concerns the attachment of a lipid group to the amino
acid cysteine, the consequence of which is that the lipid plugs into the
membrane, thus creating a fourth intracellular loop.
The agonists for class A are usually quite
small and their binding sites are often deep in the transmembrane region
(e.g. binding of noradrenaline to the
beta-adrenergic receptor). Most peptide hormones and neuropeptides have
receptors from the Class B family of receptors. Class B receptors have a larger N-terminal
extracellular region than class A receptors, and this region possess many
disulphide bridges. These bridges may be important in the formation of the
globular ligand-binding domain. The ligands for class B receptors can be quite
large, such as large proteins. It is thought that upon binding to the
extracellular domain the ligand is then in the proper orientation so that
another part of the ligand can induce receptor activation through interactions
with the transmembrane regions of the receptor. Class C is a very small family, consisting of receptors for only three
ligands, namely: (1) GABA, with the receptor referred to as
the GABAb receptor to distinguish it from the ionotropic GABAa receptor,
(2) glutamate, called the metabotropic
glutamate receptor to distinguish it from the ionotropic receptors for
glutamate, and
(3) calcium, called the Ca2+ sensing
receptor, which is important in the regulation of release of hormone involved in
Ca2+ homeostasis.
Class C receptors are characterized by very large
N-terminal extracellular domains. There are many disulphide bridges in this
extracellular region that are important in forming the ligand binding domain.
Ligand binding to Class C receptors is believed to induce receptor dimerization
which initiates the process of transmembrane signaling.
Interesting
points: The sequence homology between the classes of GRPCs is very low. This
has led some to suggest that the 7-transmembrane receptors have been reinvented
a number of times during evolution. The class E cAMP receptor has only been
found in the slime mold Dictyostelium. |