|
MOLECULAR & CELLULAR
NEUROBIOLOGY
Master Course Cognitive Neuroscience - Radboud
University, Nijmegen
|
|
|
|
Chapter 2: Cells and within cells |
|
|
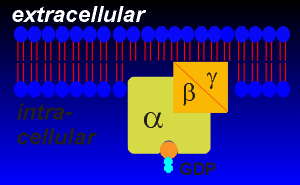
First the principles.......The principle of receptor signaling through G-proteins is that the ligand induces a change in the sturcture of the receptor such that the receptor-ligand complex can activate the G-protein. Intracellular loops of the receptors are responsible for the activation of the G-proteins. The G-proteins associated with receptor transduction are the so-called "trimeric" G-proteins because they are composed of three subunits, alpha, beta and gamma. The trimeric designation distinguishes the receptor-associated G-proteins from smaller intracellular "monomeric" G-proteins that are involved in vesicular traffic and other processes within the cell. |
|
|
They are called G-proteins because they are GTPases (they bind GTP and hydrolyze it to GDP). They are found embedded within the inner envelope of the lipid bilayer. Alpha subunits have molecular weights of ~45,000 daltons and beta and gamma subunits are around ~20 - 30,000 daltons each. The binding of GTP and its conversion to GDP is critical to the function of the G-proteins. The binding of GTP activates the G-protein for interaction with effector proteins. |
|
With the exchange of GDP for GTP the alpha and the beta/gamma subunits
fall apart, each now in an active form to activate (or inactivate)
effector proteins. The beta and gamma subunits are very tightly bound
and generally do not come apart. The exchange of GDP for GTP is the most
difficult step in the GTPase cycle of this protein (that's why the GTP
is having such difficulty with the exchange in the animation). This exchange is the rate-limiting step which determines how fast the G-protein hydrolyzes GTP to GDP. The hydrolysis of GTP to GDP returns the G-protein to the inactive state. Here the inherent GTPase activity of the alpha subunit hydrolyzes GTP to GDP. In the GDP-bound form the alpha subunit can now bind with the beta/gamma subunit and a trimeric inactive complex is formed. |
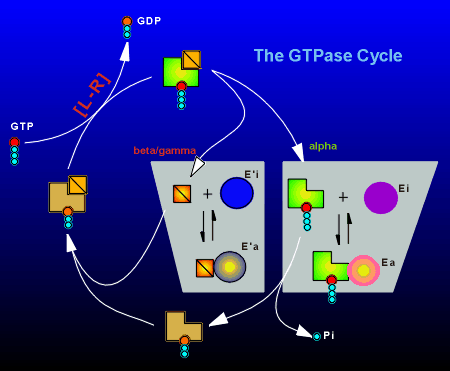
The ligand-receptor complex acts as an enzyme
|
As outlined above, the G-protein catalyzes the hydrolysis of GTP, with the rate limiting step in the GTPase cycle being the exchange of GDP for GTP. The ligand-receptor complex (indicated as [L-R] in the figure to right) facilitates this exchange process. In the active GTP-bound form the alpha subunit has two choices:
Likewise the free beta/gamma subunit can interact with an effector (E') to take it from the inactive to the active state (E'i to E'a). At rest, the bulk of the G-proteins are in the inactive GDP-bound trimeric form. |
 |
|
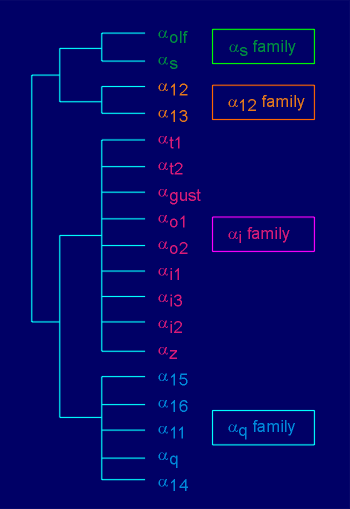
G-proteins take their name from the alpha subunit (i.e. Gs possesses the alpha(s) subunit, Gi the alpha(i) subunit, etc). There is a specificity in the substrate preference of alpha subunits. For example, alpha(s) stimulates the effector enzyme adenylyl cyclase: the "s" stands for "stimulation". Likewise, the alpha(i) inhibits adenylyl cyclase: the "i" stands for "inhibition". In general, alpha(q) stimulates the enzyme phospholipase C beta (the origins of the designation q is unclear; in earlier literature this subunit was designated "p", presumably the "p" for phospholipase C beta). There are at least 18 different alpha subunits (listed in figure to the right). On the basis of amino acid sequence homology these can be classified into 4 families, the alpha(s),alpha(12), alpha(i) and alpha(q) family. |
 |
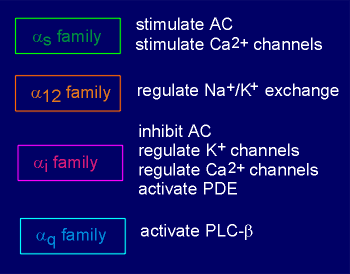 |
Alpha subunits can have more than one effector (list to left). For example, alpha(i), besides inhibiting adenylyl cyclase, has been reported to interact with and stimulate K+ channels on the membrane (thus causing hyperpolarization and inhibition of the cell). This same alpha subunit has also been reported capable of inhibiting voltage-operated Ca2+ channels on the membrane. Thus, this subunit has general inhibitory effect on the cell, whether it works through adenylyl cyclase, K+ channels, or Ca2+ channels. The closely related family member alpha(o) is also reported to act on K+ channels and probably Ca2+ channels as well. Some of the G-proteins are involved in sensory transduction (transduction of sensory information such as light, smell or taste into receptor cell potentials). For example, alpha(t), which is in the G(i) family, activates a phosphodiesterase (an enzyme for the breakdown of cyclic nucleotides) in the rod cells of the eye. |
|
Alpha(olf) is found in the olfactory system where it participates in signal transduction of odorants and alpha(gust) is found in the gustatory system where it is involved in signal transduction of taste. |
|

|
|
Beta/gamma subunits have less target specificityThere are at least 5 beta subunits and 12 gamma subunits. In general, beta/gamma dimers have much less target specicity than alpha subunits (i.e. they interact with a large spectrum of effectors). The nonspecific beta/gamma effects are perplexing since activation of all G proteins gives rise to free beta/gamma dimers. It is perplexing because it is unclear what the point is of having specific alpha targets, if the beta/gammas are acitvating everything. One possible explanation is that beta/gamma in general must be present in far higher concentrations than alpha in order to exert their effects. Thus, beta/gamma may provide a general readout for the cell of the total incoming information transmitted by G proteins. Alternatively, beta/gamma dimers may be generated in signaling domains on the membrane which possess only certain effectors (and thus inappropriate activations are impossible). |
|
 |
Finally, there is one beta/gamma target which warrents special attention, namely beta-adrenergic receptor kinase (beta-ARK), renamed G protein-coupled receptor kinase (GRK). This effector provides an internal negative feedback loop to the working of G protein receptors in general.
|
|
|
| Next page: Transcription and signalling | Go back to: G-protein coupled receptors |
|
|