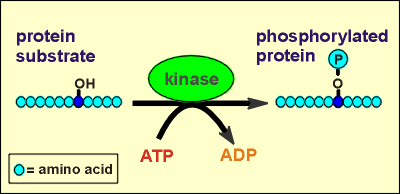
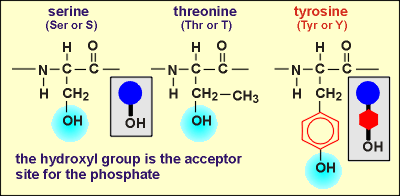
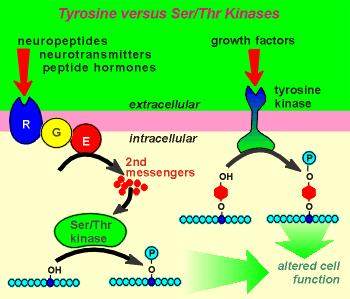
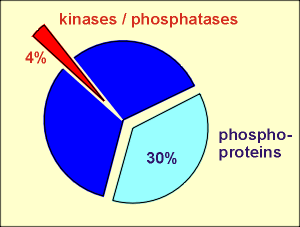
Kinases are enzymes which catalyze the phosphorylation of proteins. They do so on hydroxyl groups on the side chains of amino acids within the protein structure. ATP is the phosphate donor in this enzymatic reaction and ADP is generated in the process. The substrate proteins for the kinases are often other enzymes. Phosphorylation of the enzymes affects enzymatic activity. For some enzymes phosphorylation leads to an activation; for others, it leads to inactivation. By regulating the functioning of enzymes and other proteins in the cell the kinases are very important in regulating cellular events.