Secreted and membrane-associated proteins
As mentioned in other parts, proteins that are membrane bound or are destined for excretion are synthesized by ribosomes associated with the membranes of the rough endoplasmic reticulum (RER). This class of proteins all contain an N-terminus termed a signal sequence or signal peptide. The signal peptide is usually 13-36 predominantly hydrophobic residues, is recognized by a multi-protein complex (the signal recognition particle, SRP) and is removed following passage through the ER membrane. The removal of the signal peptide is catalyzed by signal peptidase. Proteins that contain a signal peptide are called preproteins to distinguish them from proproteins. However, some proteins that are destined for secretion are also further processed by proteolic cleavage before or following secretion and, therefore contain pro sequences. This class of proteins is termed preproproteins. If the protein is secreted, it will end up completely in the lumen of the ER. If the protein is membrane associated a stop transfer motif in the protein will stop the transfer of the protein through the ER membrane. This will become the membrane-spanning domain of the protein.
Proteolytic cleavage
Most proteins undergo proteolytic cleavage following translation. The simplest form of this is the removal of the initiation methionine. Many proteins are synthesized as inactive precursors that are activated under proper physiological conditions by limited proteolysis. Pancreatic enzymes and enzymes involved in clotting are examples of the latter. Inactive precursor proteins that are activated by removal of polypeptides are termed proproteins. A complex example of post-translational processing of a preproprotein is the cleavage of prepro-opiomelanocortin (POMC) synthesized in the pituitary. This preproprotein undergoes complex cleavages, the pathway of which differs depending upon the cellular location of POMC synthesis. Another example of a preproprotein is insulin. Since insulin is secreted from the pancreas it has a prepeptide. Following cleavage of the 24 amino acid signal peptide the protein folds into proinsulin. Proinsulin is further cleaved yielding active insulin which is composed of two peptide chains linked togehter through disulfide bonds. Still other proteins (of the enzyme class) are synthesized as inactive precursors called zymogens. Zymogens are activated by proteolytic cleavage such as is the situation for several proteins of the blood clotting cascade. For more details on post-translational modifications of prohormones, see under Protein secretion / Secretory pathway.
Acylation
Many proteins are modified at their N-termini following synthesis. In most cases the initiator methionine is hydrolyzed and an acetyl group is added to the new N-terminal amino acid. Acetyl-CoA is the acetyl donor for these reactions. Some proteins have the 14 carbon myristoyl group added to their N-termini. The donor for this modification is myristoyl-CoA. This latter modification allows association of the modified protein with membranes. The catalytic subunit of cyclicAMP-dependent protein kinase (PKA) is myristoylated.
Methylation
Post-translational methylation of proteins occurs on nitrogens and oxygens. The activated methyl donor is S-adenosylmethionine (SAM). The most common methylations are on the ε-amine of lysine residues. Methylation of lysine residues in histones in DNA is an important regulator of chromatin structure and consequently of transcriptional activity. Lysine methylation was originally thought to be a permanent covalent mark, providing long-term signaling, including the histone-dependent mechanism for transcriptional memory. However, recent evidence has shown that lysine methylation, similar to other covalent modifications, can be transient and dynamically regulated by an opposing de-methylation activity. Recent findings indicate that methylation of lysine residues affects gene expression not only at the level of chromatin, but also by modifying transcription factors. Additional nitrogen methylations (the imidazole ring of histidine, the guanidino moiety of arginine, the R-group amides of glutamate and aspartate) and oxygen methylation (the R-group carboxylates of gutamate and aspartate) are found as well.
As indicated below, many proteins are modified at their C-terminus by prenylation near a cysteine residue in the consensus CAAX. Following the prenylation reaction the protein is cleaved at the peptide bond of the cysteine and the carboxylate residue is methylated by a prenylated protein methyltransferase. One such protein that undergoes this type of modification is the proto-oncogene RAS.
Phosphorylation
Post-translational phosphorylation is one of the most common protein modifications that occurs in animal cells. The vast majority of phosphorylations occur as a mechanism to regulate the biological activity of a protein and as such are transient. In other words a phosphate (or more than one in many cases) is added and later removed. The enzymes that phosphorylate proteins are termed kinases and those that remove phosphates are termed phosphatases. Protein kinases catalyze reactions of the following type:
ATP + protein <——> phosphoprotein + ADP
In animal cells serine, threonine and tyrosine are the amino acids subject to phosphorylation. The largest group of kinases are those that phsophorylate either serines or threonines and as such are termed serine/threonine kinases. The ratio of phosphorylation of the three different amino acids is approximately 1000/100/1 for serine/threonine/tyrosine. Although the level of tyrosine phosphorylation is minor, the importance of phosphorylation of this amino acid is profound. As an example, the activity of numerous growth factor receptors is controlled by tyrosine phosphorylation.
Sulfation
Sulfate modification of proteins occurs at tyrosine residues. As many as 1% of all tyrosine residues present in the eukaryotic proteome are modified by sulfate addition making this the most common tyrosine modification. Tyrosine sulfation is accomplished via the activity of tyrosylprotein sulfotransferases (TPST) which are membrane-associated enzymes of the trans-Golgi network. Addition of sulfate occurs almost exclusively on secreted and trans-membrane spanning proteins. Since sulfate is added permanently it is necessary for the biological activity and not used as a regulatory modification like that of tyrosine phosphorylation. At least 34 human proteins have been identified that are tyrosine sulfated although the total number that are predicted is much higher. In all vertebrates a total of 310 tyrosine sulfated proteins have been identified. It is predicted that the mouse proteome is likely to contain over 2000 tyrosine sulfated proteins. The addition of sulfate to tyrosine is believed to play a role in the modulation of protein-protein interactions of secreted and membrane-bound proteins. The process of tyrosine sulfation has been shown to be critical for the processes of blood coagulation, various immune functions, intracellular trafficking, and ligand recognition by several G-protein-coupled receptors (GPCRs). Some well-known tyrosine sulfated proteins are the coagulation protein factor VIII, and the gut peptides gastrin and cholecystokinin (CCK).
Prenylation
Prenylation refers to the addition of the 15 carbon farnesyl group or the 20 carbon geranylgeranyl group to acceptor proteins, both of which are isoprenoid compounds derived from the cholesterol biosynthetic pathway. The isoprenoid groups are attached to cysteine residues at the carboxy terminus of proteins in a thioether linkage (C-S-C). A common consensus sequence at the C-terminus of prenylated proteins has been identified and is composed of CAAX, where C is cysteine, A is any aliphatic amino acid (except alanine) and X is the C-terminal amino acid. In order for the prenylation reaction to occur the three C-terminal amino acids (AAX) are first removed. Following attachment of the prenyl group the carboxylate of the cysteine is methylated.
In addition to numerous prenylated proteins that contain the CAAX consensus, prenylation is known to occur on proteins of the RAB family of RAS-related G-proteins. There are at least 60 proteins in this family that are prenylated at either a CC or CXC element in their C-termini. The RAB family of proteins are involved in signaling pathways that control intracellular membrane trafficking. Some of the most important proteins whose functions depend upon prenylation are those that modulate immune responses. These include proteins involved in leukocyte motility, activation, and proliferation and endothelial cell immune functions. Other important examples of prenylated proteins include the oncogenic GTP-binding and hydrolyzing protein RAS and the γ-subunit of the visual protein transducin, both of which are farnesylated. In addition, numerous GTP-binding and hydrolyzing proteins (termed G-proteins) of signal transduction cascades have γ-subunits modified by geranylgeranylation.
Amidation
The donor of the amide for C-terminal amidation is glycine. Several peptide hormones such as oxytocin and vasopressin have C-terminal amidation.
Glycosylation
See under Protein secretion / Secretory pathway (the Golgi complex).
Ubiquitin, process of ubiquitination and proteosome-mediated targeted protein degradation (see also under Protein degradation in the cell)
Proteins are in a continual state of flux, being synthesized and degraded. In addition, when proteins become damaged they must be degraded to prevent aberrant activities of the defective proteins and/or other proteins associated with those that have been damaged. One of the major mechanisms for the destruction of cellular proteins involves a complex structure referred to as the proteosome. In eukaryotic cells the proteosome is found in the cytosol and the nucleus and has a large mass. Degradation of proteins in the proteosome occurs via an ATP-dependent mechanism. Proteins that are to be degraded by the proteosome are first tagged by attachment of multimers of the 76 amino acid polypeptide ubiquitin. Many proteins involved in cell cycle regulation, control of proliferation and differentiation, programmed cell death (apoptosis), DNA repair, immune and inflammatory processes and organelle biogenesis have been discovered to undergo regulated degradation via the proteosome. Of clinical significance are the recent findings that deregulation of the functions of the proteosome can contribute to the pathogenesis of various human diseases such as cancer, myeloproliferative diseases, and neurodegenerative diseases.
The degradation of proteins via the proteosome involves a two-step process starting with ubiquitination of the protein followed by entry into and degradation by the proteosome complex with release of ubiquitin monomers that can be re-used to tag additional proteins. The process of ubiquitin addition to the substrate protein involves multiple ubiquitin additions such that the targeted protein is polyubiquitinated. Attachment of ubiquitin involves a series of three enzyme activities. The first, identified as E1 (also called ubiquitin-activating enzymes), activates ubiquitin in an ATP-dependent manner such that the ubiquitin is bound to the E1 enzyme via a high-energy thiol ester. The next class of enzyme, referred to as E2 (also called ubiquitin-conjugating enzymes or ubiquitin-carrier proteins), transfers the ubiquitin via an E2 thiol ester intermediate to the substrate protein. The substrate proteins are recognizable by E2 because they are bound by the third class of enzyme called E3 or ubiquitin-protein ligases. The E3 enzymes carry out the final step in the process which is the covalent attachment of ubiquitin to the substrate protein. The ubiquitin is generally transferred to the ε-amino group of an internal lysine residue in the substrate protein. There are however, examples where the ubiquitin is attached to the N-terminal amino group in a substrate protein. Whereas, ubiquitination targets proteins for degradation in the proteosome there needs to be a mechanism to ensure that inappropriately tagged proteins, i.e. those that are not destined for degradation, can be untagged. There are a family of enzymes called isopeptidases that carry out this vital function of removing ubiquitin from from proteins to which it is mistakenly attached.
Inactivation of a critical activity such as that catalyzed by the E1 enzymes results in lethality. However, there are numerous pathological states that can be attributed, in part, to mutations in recognition motifs in ubiquitination substrates and enzymes in the ubiquitination process. Disease states associated with the ubiquitin modification system can be classified into two groups. One group results from a loss of function mutation in a ubiquitin system enzyme or target protein that results in stabilization of the protein that should normally be degraded. The other group results from gain of function mutations that result in abnormal or accelerated degradation of target proteins. The most obvious disease state that could be expected to arise as a result of defective ubiquitination processes is cancer. In fact, many malignancies are known to result from defective ubiquitin-mediated degradation of growth-promoting proteins such as FOS, MYC, and SRC. Likewise, inappropriate degradation of key regulators of cell cycle progression such as the tumor suppressor p53 and p27KIP1 (CDKN1B) is also associated with various types of cancer. In addition to cancers, defective ubiquitination is found associated with neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, and amyotrophic lateral sclerosis (ALS).
Protein modification by SUMO
As discussed in the above section, many proteins are post-translationally modified via the addition of ubiquitin. Over the past several years numerous ubiquitin-like (Ubl) proteins have been identified. Like ubiquitin, Ubls are added to other proteins via post-translational reactions. However, unlike ubiquitination which targets proteins for degradation in the proteosome, Ubl modifications do not. Although there are several types of Ubls, those with the broadest range of functions and the largest number of known substrates are members of the SUMO (small ubiquitin-related modifier) proteins. There have been over 50 proteins, that function in a variety of different capacities within cells, shown to be modified by SUMOylation. Modification of proteins by SUMO addition has been shown to occur in all tissues and at all developmental stages. There are four SUMO proteins in mammalian cells designated SUMO-1 to SUMO-4.
Following de novo synthesis, SUMO proteins must undergo a C-terminal cleavage processing event in order to render the proteins biologically active. The C-terminal cleavage reactions are catalyzed by a family of proteins called SENP (sentrin/SUMO-specific protease). The removal of the C-terminal amino acids reveals a di-glycine (–G–G) motif that allows the SUMO protein to subsequently conjugate to lysine (K) residues in target proteins. SUMO conjugation to target proteins requires a series of enzymatic steps that is similar in mechanism to the conjugation of ubiquitin to target proteins. The consensus motif for SUMOylation is the following: ΨKxD/E where Ψ is a large hydrophobic amino acid and x is any amino acid. Approximately 75% of all SUMOylated proteins contain this target motif, however, not all proteins that contain this motif are SUMOylated and some proteins are SUMOylated on lysine residues that do not lie in this motif.
Whereas, ubiquitination of a protein results in its destruction in the proteosome, SUMOylation is a dynamic process and once attached the SUMO residue can be removed. Removal of SUMO from a target protein is accomplished by the same SENP enzymes that are required for the activation step of SUMO processing. The exact functional consequences of SUMOylation of a particular target protein is difficult to predict. However, modification of target proteins by SUMO addition is likely to lead to at least three non-mutually exclusive effects. The attachment of SUMO can result in the masking of a site in the target protein that is required for binding or interaction with a substrate protein. The addition of SUMO may alternatively result in the formation of an attachment site allowing for the recruitment of proteins that can now interact with the SUMOylated protein. The third consequence could be that the conformation of the SUMOylated protein is altered such that activity is regulated in some way.
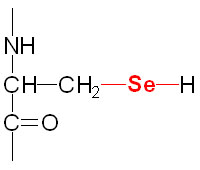 Structure
of the selenocysteine residue
Structure
of the selenocysteine residue