miRNAs and the brain
miRNAs in the central nervous system (CNS)
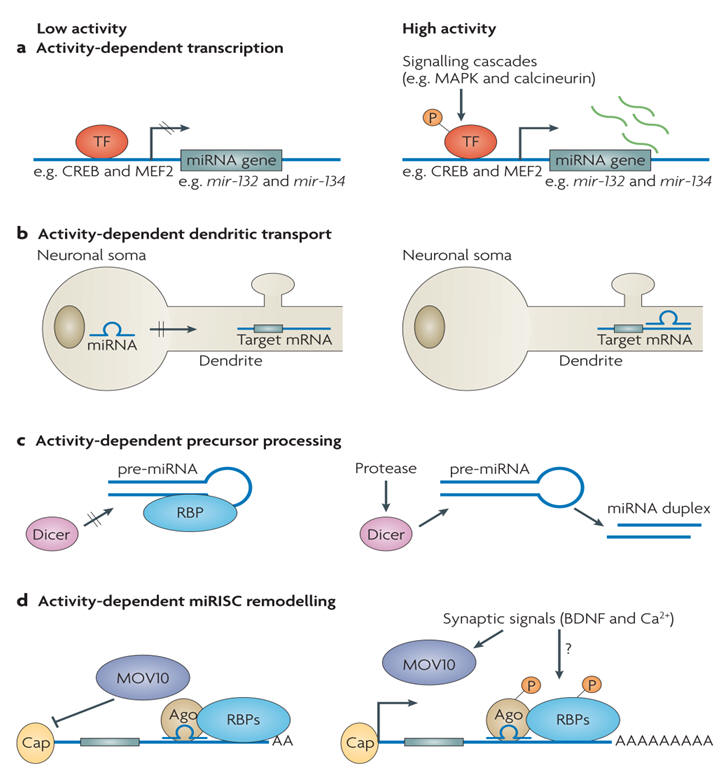
Over half of the miRNAs so far identified are expressed predominantly or exclusively in the brain. The role of miRNAs in brain function is only just emerging. There is accumulating evidence to indicate that miRNAs play a key role in the development of the CNS and play an important role in neuronal differentiation (see below). There is now evidence that miRNAs also play a role in controlling neurotransmitter release.
A major challenge is the prediction of mammalian miRNA targets: thousands of human genes may be miRNA targets. Interactions between miRNA and their targets are often classified as a “switch,” “tuning,” or “neutral”. A “switch” interaction is characterized by a miRNA reducing the target protein activity to a negligible level. This differs from “tuning” interactions, which result in the fine modulation of target protein levels to an optimal range for a given physiological and/or developmental state. “Neutral” targets are usually species-specific interactions that have neither a positive nor negative effect on the cell.
miRNAs and neurodevelopment
The human transcriptome shows an amazing complexity with major posttranscriptional control of neuronal development by miRNAs. In the nervous system, critical roles for a number of miRNAs in neuronal development or function have been demonstrated in several model organisms (e.g. Lsy-6 and miR-273 in C. elegans, miR-7 in Drosophila, and miR-134, miR-132, miR-138 and members of the miR-200 family in mammals). In particular, miR-9 and miR-124 are specifically expressed in the mammalian nervous system, and their respective nucleotide sequences are 100% identical among many species. Yet, their expression patterns and mRNA targets are less conserved throughout evolution. As a consequence, these miRNAs exhibit diverse context-dependent functions in different aspects of neuronal development, ranging from early neurogenesis and neuronal differentiation to dendritic morphogenesis and synaptic plasticity (see figure below).
miR-9 and miR-124:
- - are the most extensively studied neuronal miRNAs
- - are among the most ancient animal miRNAs that show cell-type specific expression
- - - are implicated in multiple stages of neuronal development
- - may play key roles in the development of new body plans
- - their roles in various aspects of neuronal development in different species will serve as an excellent case study to elucidate the functional conservation and divergence of neuronal miRNAs during evolution
Some other neuronal miRNAs also exhibit context-dependent functions in development. Thus, post-transcriptional regulation of spatial and temporal expression levels of protein-coding genes by miRNAs contributes uniquely to the proper development and evolution of the complex nervous system.
A recurring theme seems to be that one or a few mRNA targets account for the majority of the phenotype in a particular developmental or cellular process. This is likely the case for miRNAs as well. The context-dependent functions of miRNAs in neuronal development or other processes could be explained in part by the variations in transcriptome composition in diverse cell types in different species. The ratio of copy numbers between a specific miRNA and its target may also influence its developmental functions. Thus, it will be useful to systematically identify context-dependent targets of a specific miRNA and to study the endogenous activities of specific miRNAs in their physiological contexts. Overall, conserved neuronal miRNAs may assume novel functions, which, together with newly evolved miRNAs, such as those uniquely expressed in the human brain, may contribute to the evolution of this organ.
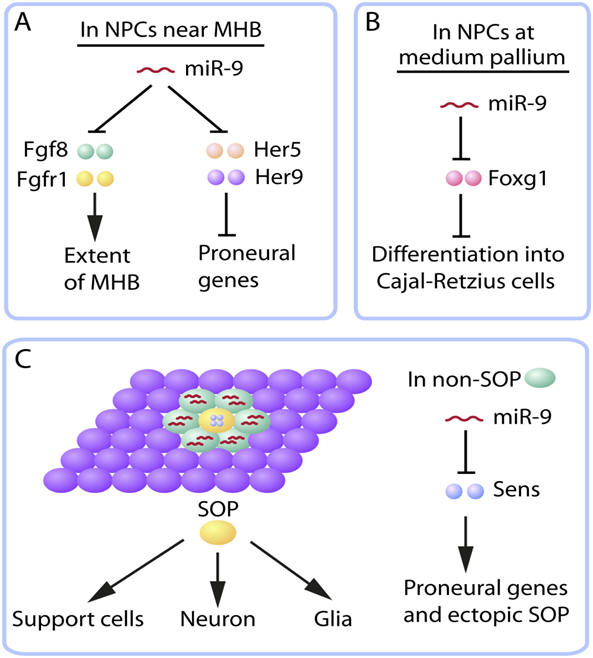
Context-dependent functions of miR-9 in neurogenesis. (A,B) In the developing brains of zebrafish (A) and mice (B), miR-9 is expressed in neural progenitor cells (NPCs) and promotes neurogenesis by downregulating different suppressors of neuronal differentiation. (C) During early neurogenesis in Drosophila embryos, miR-9 is not expressed in sensory organ precursors (SOPs) that eventually give rise to sensory neurons and other cell types. Instead, it is expressed in non-SOP cells, including those adjacent to the SOP in the pro-neural cluster, to suppress the residual expression of Sens, an activator of proneural genes in the process of lateral inhibition. Fgf, fibroblast growth factor; Fgfr, fibroblast growth factor receptor; Foxg1, forkhead box protein G1; MHB, midbrain-hindbrain boundary.