|
As mentioned under "Transcription", gene expression occurs in two steps:
-
transcription of the information encoded in DNA into a molecule of RNA (see
under "Transcription") and
-
translation of the information encoded in the nucleotides of mRNA into a defined sequence of amino acids in a protein (i.e.
RNA-directed synthesis of polypeptides, described
here).
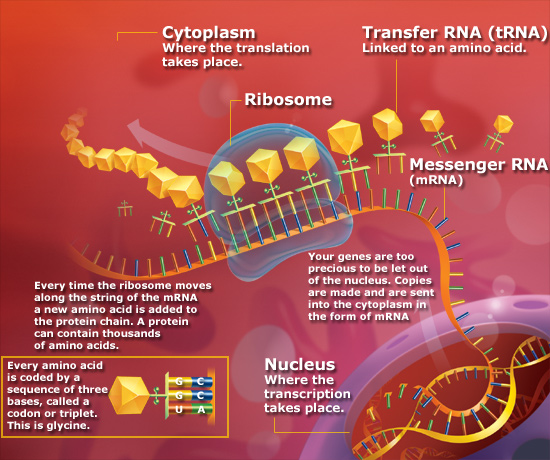
In eukaryotes, the processes of transcription and translation are separated both spatially and in time. Transcription of DNA into mRNA occurs in the nucleus. Translation of mRNA into polypeptides occurs on polysomes in the cytoplasm. In prokaryotes (which have no nucleus), both
of these steps of gene expression occur simultaneously: the nascent mRNA molecule begins to be translated even before its transcription from DNA is complete.
Although the chemistry of peptide bond formation is relatively simple, the
processes leading to the ability to form a peptide bond are exceedingly complex.
Translation requires all three classes of RNA. The template for correct addition
of individual amino acids is the mRNA, yet both transfer RNAs (tRNAs) and
ribosomal RNAs (rRNAs) are involved in the process.
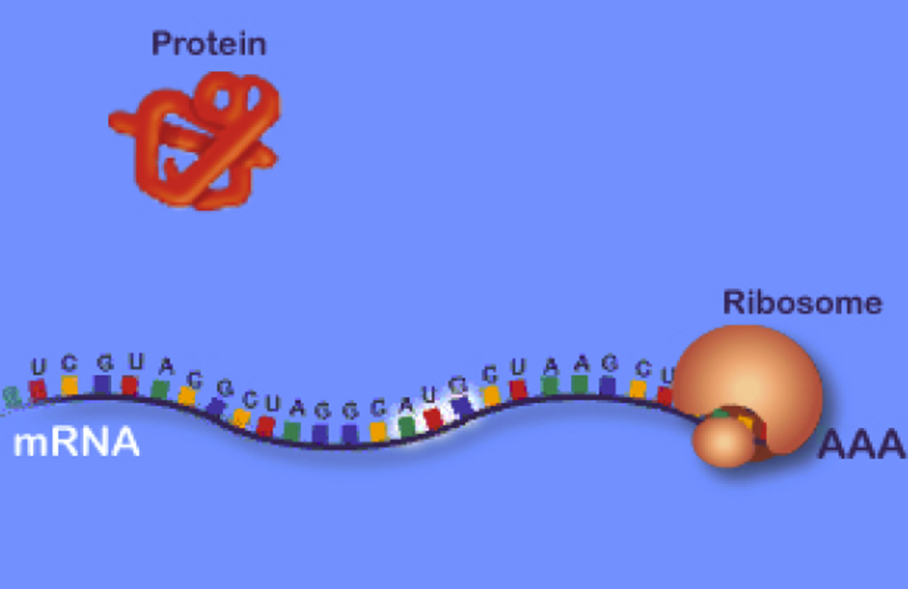
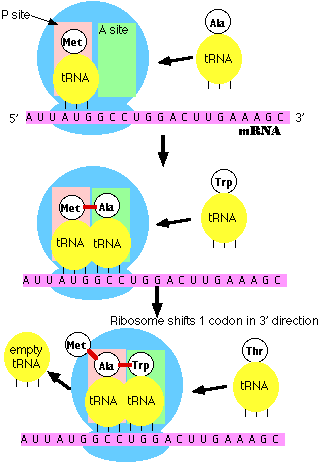
How does a particular sequence of nucleotides specify a particular sequence of amino acids?
The answer: by means of the tRNA molecules, each specific for one amino acid and for a particular triplet of nucleotides in mRNA called a codon. The family of tRNA molecules enables the codons in an mRNA molecule to be translated into the sequence of amino acids in the protein. Translation is
thus the part of protein synthesis where the ribosomes in the
cytoplasm use tRNA to attach to the mRNA and translate the
bases into amino acids and tRNA molecules bring the specified amino acids that the
ribosome links together to make a protein.Thus, the tRNAs carry activated
amino acids into the ribosome which is composed of rRNA and ribosomal
proteins. The ribosome is associated with the mRNA ensuring correct
access of activated tRNAs and containing the necessary enzymatic
activities to catalyze peptide bond formation.
Protein translation thus occurs when a single mRNA moves along the ribosome and is
read by tRNA molecules, which bring the amino acid to the chain. Once a
polypeptide is formed from the specific mRNA, it may in itself form the protein
or combine with other polypeptides to form the mature protein. Once the protein
is formed, it has certain amino acid sequences which direct it to its specific
compartment in the cell (see below for a movie
on protein transport into the mitochondrion). Secreted protein molecules, for example, contain a
hydrophobic tail sequence which directs it to the endoplasmic reticulum
membrane. The proteins perform all of the work of the cell and are each
synthesized from a unique mRNA. Gene expression refers to the whole process from
the formation of the gene to a mature protein. Many proteins undergo chemical
modification posttranslationnally, such as glycosylation. This form of
posttranslational modification is particularly common with cellular secretory or
membrane proteins. It may involve the development of disulfide bonds,
proteolytic cleavage of the newly synthesized protein, or the addition of
carbohydrate moieties. These posttranslational changes may importantly affect
function and subcellular localization of proteins.
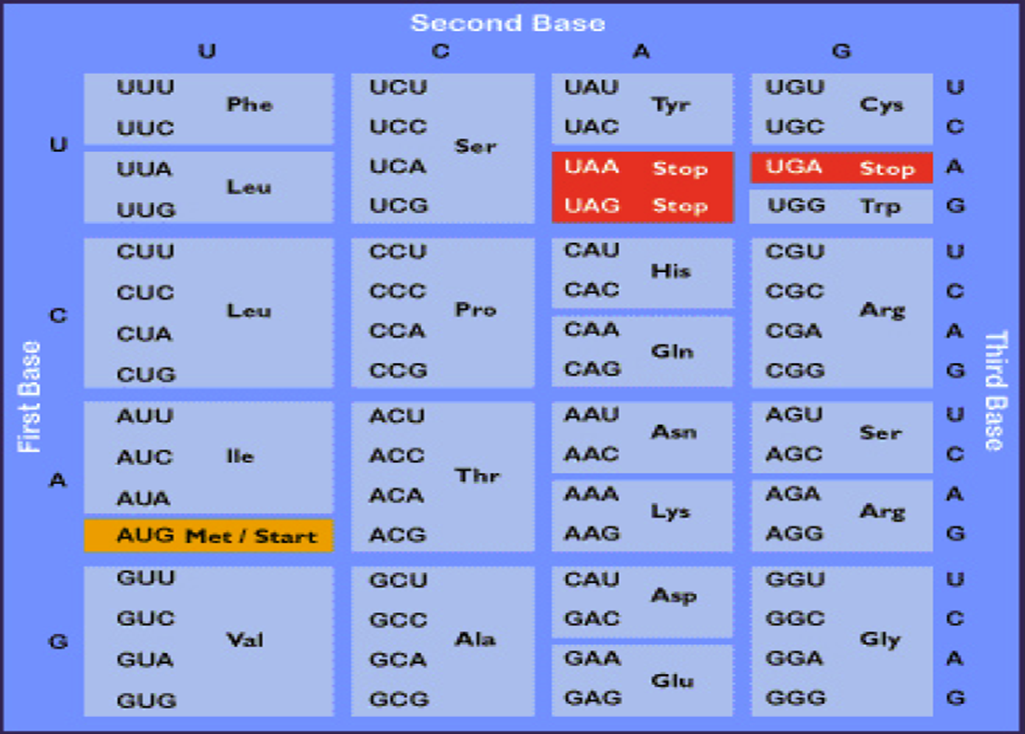
In sum, the genetic code is
read in a sequential manner starting near the 5' end of the mRNA.
This means that translation proceeds along the mRNA in the 5'
——> 3' direction which corresponds to the N-terminal to
C-terminal direction of the amino acid sequences within proteins.
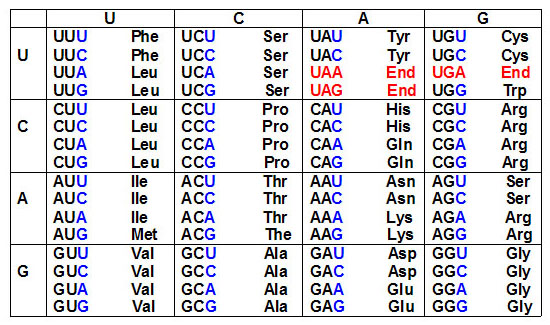
The code is composed of a triplet of nucleotides. All 64
possible combinations of the four nucleotides code for amino acids,
i.e. the code is degenerate since there are only 20 amino acids.
The precise dictionary of the genetic code was determined with
the use of in vitro translation systems and
polyribonucleotides. The results of these experiments confirmed
that some amino acids are encoded by more than one triplet
codon, hence the degeneracy of the genetic code. These
experiments also established the identity of translational
termination codons.
|