|
Ion channels in action
There are two major classes of ion channels, those that are
induced to open through the binding of a ligand and those that are induced to
open by a change in membrane potential. The first group are referred to as
ligand-operated or ligand-gated ion channels. The second group are referred to
as voltage-operated or voltage-gated ion channels. On the right is a side view
through the middle of a ligand-operated channel. The ligand (green circle) binds
to specific sites in extracellular side of the ion channel. As a consequence of
this binding "gates" deep within the receptor are induced to open and thus ions
can flow through the channel. Ligands are usually small molecules
neurotransmitters such as acetylcholine, glutamic acid or gamma aminobutyric
acid (GABA).
For
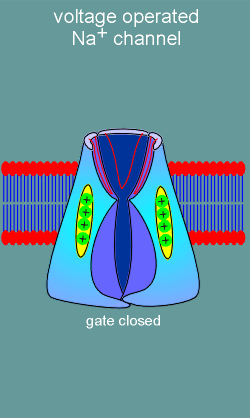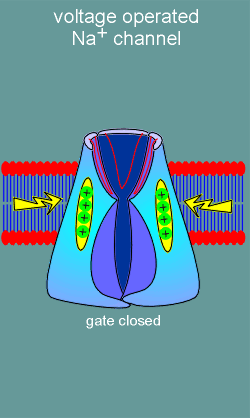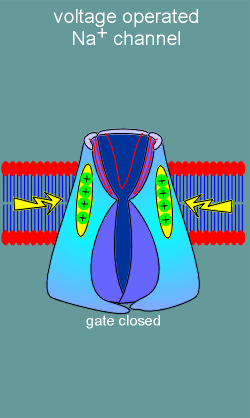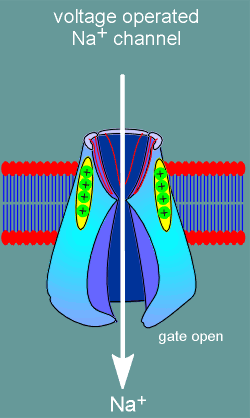
the second major class of ion channels, the voltage operated channels, there are
no ligand binding sites. Rather, these ion channels possess voltage sensors
(structure with positive charges in figure to left). With membrane
depolarization (yellow arrows) the voltage sensors move and, in doing so, induce
opening of an ion gate on the intracellular side of the channel. Ions are now
free to flow into or out of the cell (in this example the channel is a Na+
channel and thus Na+ flows into the cell).
Ion channels are constructed from discrete
proteins called "subunits". Voltage-operated channels are generally constructed
from 4 subunits whereas ligand-operated ion channels are constructed from 5
subunits. Shown is a representation of voltage-operated and ligand-operated
channels where each subunit is represented by a cylinder in the lipid bilayer
(blue circles and red tails represent phospholipids of the bilayer). By
looking straight down on the channel, depicted on the far right, you can get an
impression of the size of the pore (indicated as white circle). Note that
because the ligand-operated channel has more subunits its pore is larger
than that of a voltage-operated channel. Because of the larger pore the ligand-operated
channel is, in general, less ion specific that the voltage-operated channel. For
example, one of the very important ligand-operated ion channels is the so-called
NMDA receptor. This receptor is a Ca2+ channel but a considerable amount of Na+
also enters the cell via the receptor when the channel has opened.
|

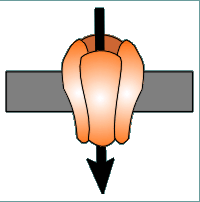

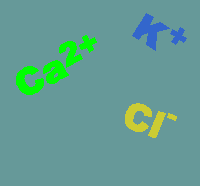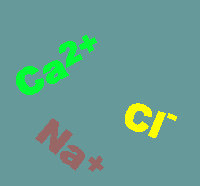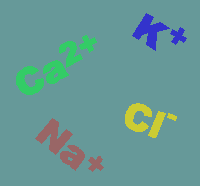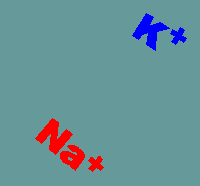 on channels are usually quite specific for the
ion which they allow to pass through the pore. Thus we speak of Ca2+
channels, Na+ channels, K+ channels and Cl-
channels. Ion specificity is largely
determined by ion filters constructed within the channels. The
direction an ion goes (into or out of the cell) depends on the so-called
electrochemical equilibrium point for the ion. The electrochemical equilibrium
takes into account both the concentration gradient and the charge of the ion.
The ion will flow into or out of the cell until it reaches its electrochemical
equilibrium point. To explain further it is important to remember that a cell
normally has a resting potential of between -50 to -70 mV. Thus, positive
charges tend to be pulled into the cell and negative charges pulled out (this is
the "electro" part of the electrochemical equilibrium point). However, for the K+
ion the concentration of the ion is far higher in the cell than outside and
thus, with opening of a K+ channel the ion flows out. This is why it
is important to consider both the charge and the concentration (i.e. the
electrochemical equilibrium point of the ion) when deciding which way an ion
will travel. Shown below are the usual directions of flow for various ions (the
intensity of the colors gives an impression of the concentration gradients
between the extracellular (top) and intracellular (bottom) compartments.
on channels are usually quite specific for the
ion which they allow to pass through the pore. Thus we speak of Ca2+
channels, Na+ channels, K+ channels and Cl-
channels. Ion specificity is largely
determined by ion filters constructed within the channels. The
direction an ion goes (into or out of the cell) depends on the so-called
electrochemical equilibrium point for the ion. The electrochemical equilibrium
takes into account both the concentration gradient and the charge of the ion.
The ion will flow into or out of the cell until it reaches its electrochemical
equilibrium point. To explain further it is important to remember that a cell
normally has a resting potential of between -50 to -70 mV. Thus, positive
charges tend to be pulled into the cell and negative charges pulled out (this is
the "electro" part of the electrochemical equilibrium point). However, for the K+
ion the concentration of the ion is far higher in the cell than outside and
thus, with opening of a K+ channel the ion flows out. This is why it
is important to consider both the charge and the concentration (i.e. the
electrochemical equilibrium point of the ion) when deciding which way an ion
will travel. Shown below are the usual directions of flow for various ions (the
intensity of the colors gives an impression of the concentration gradients
between the extracellular (top) and intracellular (bottom) compartments.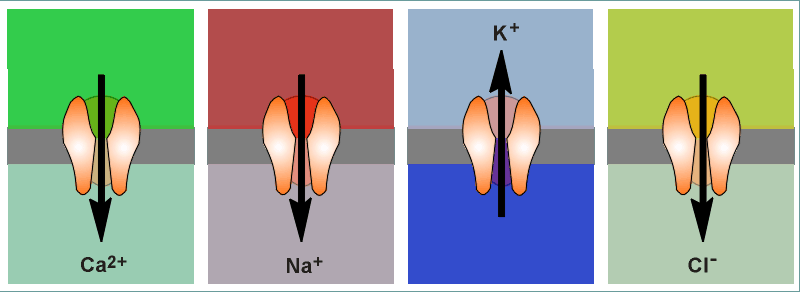
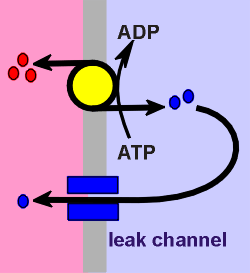 What keeps ions from slowly diffusing (leaking) to
their electrochemical equilibrium point and thus the cell loosing its resting
membrane potential? The answer, nothing!...... and thus the cell must be
constantly pumping ions to maintain the electrochemical gradients. As
an example, consider Na+ and K+, the two ions that make the most important
contribution in determining the membrane potential. Na+/K+ ATPases are
constantly active in the membrane, pumping 3 Na+ ions out for every 2 K+ ions in
(red and blue circles respectively in figure to left). This already makes the
inside of the cell more negative than the outside (3 + charges pumped out for
every two + charges pumped in). Additionally, there are so-called "K+ leak
channels" on the membrane which allow some of the K+ ions to leak out (down the
electrochemical gradient). This leakage makes the cell even more negative inside
and thus we arrive at a rest potential of -50 to -70 mV. All this pumping costs
energy, energy derived from ATP-hydrolysis. Thus, the name for this pump is
Na+/K+-ATPase.
What keeps ions from slowly diffusing (leaking) to
their electrochemical equilibrium point and thus the cell loosing its resting
membrane potential? The answer, nothing!...... and thus the cell must be
constantly pumping ions to maintain the electrochemical gradients. As
an example, consider Na+ and K+, the two ions that make the most important
contribution in determining the membrane potential. Na+/K+ ATPases are
constantly active in the membrane, pumping 3 Na+ ions out for every 2 K+ ions in
(red and blue circles respectively in figure to left). This already makes the
inside of the cell more negative than the outside (3 + charges pumped out for
every two + charges pumped in). Additionally, there are so-called "K+ leak
channels" on the membrane which allow some of the K+ ions to leak out (down the
electrochemical gradient). This leakage makes the cell even more negative inside
and thus we arrive at a rest potential of -50 to -70 mV. All this pumping costs
energy, energy derived from ATP-hydrolysis. Thus, the name for this pump is
Na+/K+-ATPase.