Changes to DNA and its associated proteins can alter gene expression without altering the DNA sequence. DNA does not exist as naked molecules in the cell: it is associated with proteins called histones to form a complex substance known as chromatin; chromatin supports DNA, but also controls access to it. Chemical modifications to the DNA or the histones alter the structure of the chromatin without changing the nucleotide sequence of the DNA. Such modifications are described as epigenetic. Epigenetics is therefore defined as: heritable changes in gene expression that occur without a change in DNA sequence. Epigenetics may involve: methylation of DNA at cytosine residues; chromatin remodeling; phosphorylation, acetylation and methylation of histone proteins; the modification and assembly of regulatory protein complexes on DNA. The epigenetic component of a disease should be easier to modify with medicine than the DNA sequence.
|
Chromatin and chromatin remodelling
Chromatin Histone modifications
|
|
Organization of DNA within the chromatin structure
Figure 1. The lowest level of organization is the nucleosome, in which two superhelical turns of DNA are wound around the outside of a histone octamer. Nucleosomes are connected to one another by short stretches of linker DNA. At the next level of organization the string of nucleosomes is folded into a fiber and these fibers are then further folded into higher-order structures. |
Click DNA packaging for a movie.
Chromatin is thus the complex of DNA, histones and nonhistone proteins in the cell nucleus. Remodelling of chromatin is a dynamic process that modulates gene expression. The fundamental unit of chromatin is the nucleosome, which consists of ~147 base pairs of DNA wrapped around a core histone octamer (~1.65 turns). Each octamer contains two copies each of the histones H2A, H2B, H3 and H4 (Figs. 1 and 2a). The nucleosomal structure of chromatin allows DNA to be tightly packaged into the nucleus by organized folding. Intricate chromatin remodelling mechanisms ensure that DNA remains accessible to the transcriptional machinery.
Changes to the structure of the chromatin have a profound influence on gene expression: if the chromatin is condensed, the factors involved in gene expression cannot get to the DNA, and the genes will be switched off. Conversely, if the chromatin is ‘open’, the genes can be switched on if required. While many heritable disorders in humans are caused by DNA sequence changes (mutations) that abolish gene expression, a number of human diseases are caused by inappropriate gene silencing, brought about by epigenetic modifications. Indeed, most cancers involve the epigenetic silencing of genes that normally control cell proliferation. In simplified terms, chromatin thus exists in an inactive, condensed state (heterochromatin), which does not allow transcription of genes, and in an activated, open state (euchromatin), which allows individual genes to be transcribed (Fig. 2b). The opening of chromatin is associated with acetylation of nearby histones, although it remains unclear whether acetylation mediates or reflects chromatin decondensation. In reality, chromatin can exist in many states in between these two extremes (Fig. 2b). Portions of chromatin are highly repressed, owing to DNA and histone methylation and the binding of repressor proteins, and might never be accessible for transcription. Other portions of chromatin are in repressed or permissive states; their basal activity is low owing to histone methylation and perhaps other modifications, but the genes are available for derepression and activation in response to transcription factors and transcriptional co-activators. Chromatin remodelling modulates gene expression with high temporal and spatial resolution by permitting small groups of nucleosomes to become more or less open, which consequently enhances or inhibits access of the transcriptional machinery to specific promoter regions.
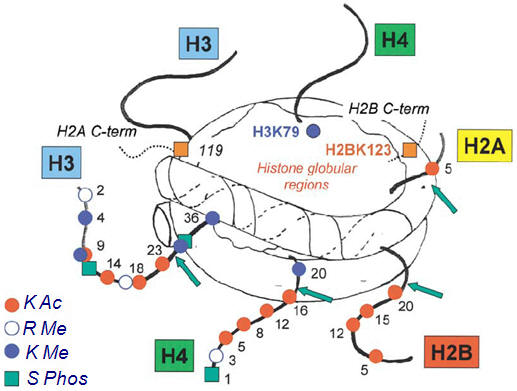
By far the best characterized chromatin remodeling mechanism in the brain is the post-translational, covalent modification of histones at distinct amino acid residues on their amino (N)-terminal tails. Such modifications include acetylation, ubiquitylation or SUMOylation at lysine (K) residues, methylation at lysine or arginine (R) residues, phosphorylation at serine (S) or threonine (T) residues, and ADP-ribosylation at glutamate (E) residues (Fig. 2c). Hyperacetylation is generally thought to promote decondensation of chromatin and an increase in gene activity, whereas hypoacetylation marks condensation and decreased activity. It has also been proposed that increased gene activity is best associated not with the level of acetylation, but with the dynamic cycling of acetylation and deacetylation. In contrast to acetylation, histone methylation can correlate with either gene activation or repression, depending on the residue undergoing methylation. Phosphorylation of histones is also associated with chromatin inhibition or activation. The roles of histone ubiquitylation, SUMOylation and ADP ribosylation are less well understood. The diversity of histone modifications supports the ‘histone code hypothesis’, which posits that the sum of modifications at a particular promoter region defines a specific epigenetic state of gene activation or silencing.
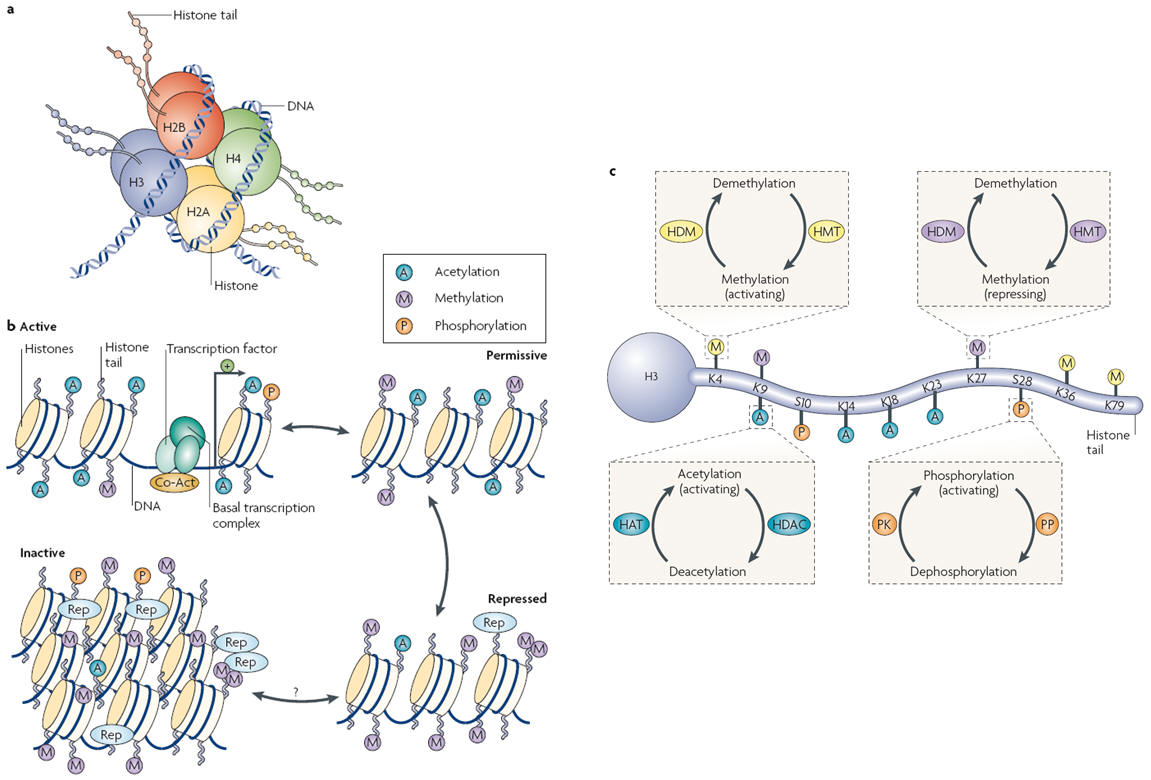
Figure 2. General scheme of chromatin remodelling. a | Picture of a nucleosome showing a DNA strand wrapped around a histone octamer composed of two copies each of the histones H2A, H2B, H3 and H4. The amino (N) termini of the histones face outward from the nucleosome complex. b | Chromatin can be conceptualized as existing in two primary structural states: as active, or open, euchromatin (top left) in which histone acetylation (A) is associated with opening the nucleosome to allow binding of the basal transcriptional complex and other activators of transcription; or as inactive, or condensed, heterochromatin where all gene activity is permanently silenced (bottom left). In reality, chromatin exists in a continuum of several functional states (active; permissive (top right); repressed (bottom right); and inactive). Enrichment of histone modifications such as acetylation and methylation (M) at histone N-terminal tails and related binding of transcription factors and co-activators (Co-Act) or repressors (Rep) to chromatin modulates the transcriptional state of the nucleosome. Recent evidence suggests that inactivated chromatin may in some cases be subject to reactivation in adult nerve cells, although this remains uncertain. c | Summary of common covalent modifications of H3, which include acetylation, methylation and phosphorylation (P) at several amino acid residues. H3 phosphoacetylation commonly involves phosphorylation of S10 and acetylation of K14. Acetylation is catalysed by histone acetyltransferases (HATs) and reversed by histone deacetylases (HDACs); lysine methylation (which can be either activating or repressing) is catalysed by histone methyltransferases (HMTs) and reversed by histone demethylases (HDMs); and phosphorylation is catalysed by protein kinases (PK) and reversed by protein phosphatases (PP), which have not yet been identified with certainty. K, lysine residue; S, serine residue.
Figure 3. DNA modifications that abolish gene expression. Open chromatin is characterized by non-methylated DNA and histones with acetylated tails. This allows the assembly of transcription factors and transcription by RNA polymerase. DNA methyltransferase activity results in the methylation of DNA. This may directly block binding by transcription factors and prevent transcription. It may also recruit methyl-binding domain proteins that have associated histone deacetylases. DNA methylation and histone deacetylation result in the condensation of chromatin into a compact state that is inaccessible by transcription factors.
Overview of epigenetic mechanisms
The enzymes that mediate the covalent histone modifications are becoming increasingly understood. Many histone acetyltransferases (HATs), which catalyse acetylation, have been identified. Several transcriptional activators contain intrinsic HAT activity. Histone deacetylases (HDACs) catalyse deacetylation; they also associate with several transcriptional repressors to further repress chromatin activity. The balance between the opposing activities of HATs and HDACs maintains acetylation on core histones and is thought to be an important determinant of transcription. Methylation at lysine or arginine residues is mediated by histone methyltransferases (HMTs). In general, histone lysine methylation is regarded as a more stable modification than other histone modifications, which seem to be more readily reversible, although the recent discovery of histone demethylases (HDMs) indicates that even methylation can be reversed. Several other general mechanisms of chromatin remodelling have been described, although they remain less well characterized in the nervous system.
DNA methylation is another important mechanism of gene repression. It occurs by transfer of a methyl group from S-adenosyl methionine (SAM) to cytosine residues at the so-called dinucleotide sequence CpG, and is catalysed by DNA methyltransferases (DNMTs). Methylation can directly switch off gene expression by preventing transcription factors binding to promoters. However, a more general effect is the attraction of methyl-binding domain (MBD) proteins, such as methyl-CpG-binding protein 2 (MeCP2), to methylated DNA. MBDs are associated with large protein complexes containing HDACs and HMTs, which function to chemically modify histones and change chromatin structure. Chromatin containing acetylated histones is open and accessible to transcription factors, and the genes are potentially active. Histone deacetylation causes the condensation of chromatin, making it inaccessible to transcription factors and the genes are therefore silenced.Thus, DNA methylation and histone methylation and deacetylation are intricately interconnected, each representing epigenetic hallmarks of the silenced promoter.
Although CpG sequences throughout the genome are usually heavily methylated, those at the promoter regions of genes, specifically at CpG clusters or islands, are methylated to a much lesser extent, and the amount of DNA methylation at a promoter correlates with the extent of gene inactivation. CpG islands are regions of more than 500 base pairs in size and with a GC content greater than 55%. CpG islands have been conserved during evolution because they are normally kept free of methylation. These stretches of DNA are located within the promoter regions of about 40% of mammalian genes and, when methylated, cause stable heritable transcriptional silencing. Aberrant de novo methylation of CpG islands is a hallmark of human cancers and is found early during carcinogenesis.
Signalling pathways in chromatin remodelling
We are beginning to understand how intracellular signalling in the brain regulates chromatin remodelling. The best-established mechanism involves the transcription factor cyclic AMP (cAMP)-response element binding protein (CREB). The activation of several signalling pathways involving cAMP, Ca2+ and extracellular signal regulated kinase (ERK) leads to the phosphorylation of CREB. CREB phosphorylation triggers the recruitment of CREB-binding protein (CBP), a transcriptional co-activator whose intrinsic HAT activity acetylates nearby histones, which loosens the chromatin and allows subsequent transcriptional activation. CBP is important for normal learning and memory, and mutations of CBP cause Rubinstein–Taybi syndrome, a form of mental retardation in humans.
Epigenetics in disease
Since epigenetic modification plays such an important role in cancer, novel therapeutic strategies are being developed that are based on the reversal of DNA methylation and the inhibition of histone deacetylation. These are being combined with new technologies to rapidly screen the genome for DNA methylation and histone acetylation patterns (epigenomics). However, diseases can also be caused by inappropriate gene activation. One example is Burkitt’s lymphoma, which is caused by the overactivity of a gene called MYC. In this case, the gene is normally found in repressed chromatin and is expressed at a low level. Its function is to promote cell proliferation. Certain abnormal chromosome rearrangements occurring in lymphocytes move this gene into a region of open and active chromatin, causing the production of large amounts of the protein. The result is the uncontrolled proliferation of lymphocytes, resulting in lymphoma. The functional significance of DNA methylation is best established in X chromosome inactivation and genetic imprinting; abnormal imprinting can lead to neurodevelopmental diseases (Table 1). More recently, DNA methylation has been implicated in the regulation of gene activity in the adult brain under normal and pathological conditions (see below).
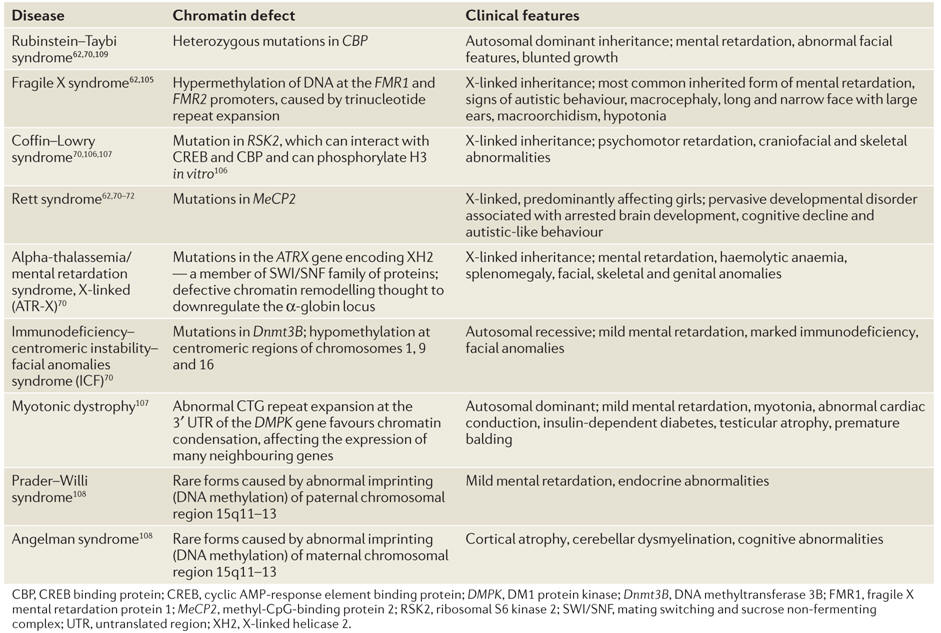
Table 1. Examples of diseases of chromatin remodelling.
The study of human disease has focused on genetic mechanisms, but disruption of the balance of epigenetic networks can cause several major pathologies, including cancer, syndromes involving chromosomal instabilities, and mental retardation. The development of new diagnostic tools might reveal other diseases that are caused by epigenetic alterations. Great potential lies in the development of ‘epigenetic therapies’ — several inhibitors of enzymes controlling epigenetic modifications, specifically DNA methyltransferases and histone deacetylases, have shown promising antitumorigenic effects for some malignancies (see below).
Mutations in genes that affect global epigenetic profiles can give rise to human diseases, which can be inherited or somatically acquired (Table 1). Interestingly, many of these epigenetic abnormalities result in chromosomal alterations and learning disabilities. The fact that all of these diseases show gross chromosomal anomalies points to a central role for epigenetic mechanisms in chromosome architecture.
Particular interest has been generated by the finding that one of the most common forms of intellectual disability in young girls, namely Rett syndrome, is due to germline mutations of the MeCP2 gene. As mentioned above, the MeCP2 protein is a methyl-binding domain protein (MBD) that binds to methylcytosine residues and the disease pathogenesis might be linked to the derepression of genes normally suppressed by DNA methylation. MeCP2 might have a key role in the control of neuronal gene activity resulting in the pathology of Rett syndrome.