|
MOLECULAR & CELLULAR
NEUROBIOLOGY
Master Course Cognitive Neuroscience - Radboud
University, Nijmegen
|
|
|
|
Chapter 2: Cells and within cells |
|
|
Neurons
| The incredibly complex connectivity between billions of neurons is necessary for the fast processing and transmission of cellular signals in the brain. Communication between a variety of neuron types underlies all basic and higher-order information processing essential for normal brain function. The ability of neural circuits to strengthen or weaken their connectivity forms a molecular basis underlying the experience-dependent changes in adaptive behaviors. |
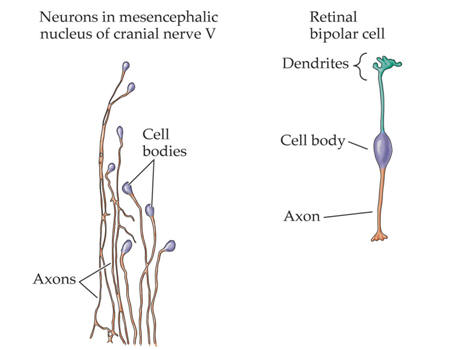
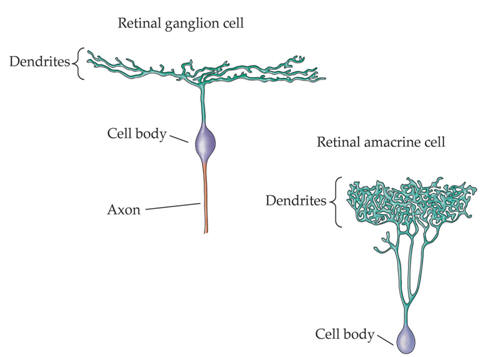
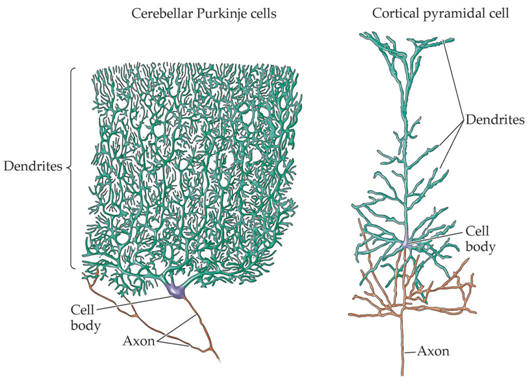
Examples of the rich variety of nerve cell morphologies
 |
 |
 |
Pyramidal neurons
|
A common class of neurons are the pyramidal neurons found in the cerebral cortex of virtually every mammal, as well as in birds, fish and reptiles. Pyramidal neurons are also common in subcortical structures such as the hippocampus and the amygdala. They are named for their shape: typically they have a soma (cell body) that is shaped like a teardrop or rounded pyramid. They also tend to have a conical spray of longer dendrites that emerge from the pointy end of the soma (apical dendrites) and a cluster of shorter dendrites that emerge from the rounded end (basal dendrites) (see figure).
|
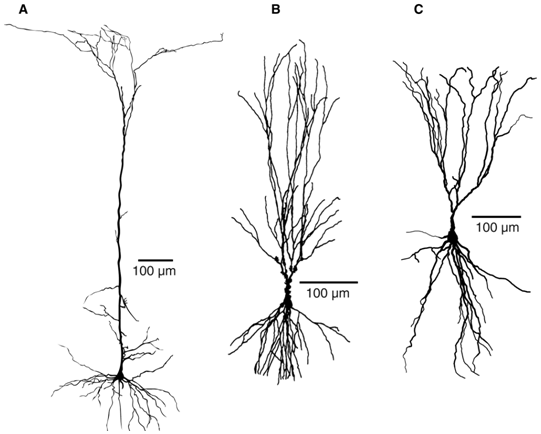 |
Dendritic morphologies of typical pyramidal neurons in different brain regions. (A) Layer 5 pyramidal neuron in the rat somatosensory cortex. (B) Pyramidal neuron in area CA3 of the rat hippocampus. (C) Layer 2 pyramidal neuron in the primary olfactory cortex of the mouse. |
| Why are pyramidal neurons important?
There are two dominant families of neurons in the cortex, excitatory
neurons, which release the neurotransmitter glutamate, and inhibitory
neurons, which release
What do pyramidal neurons do?
Like many other types of neuron, their main job is to transform synaptic
inputs into a patterned output of action potentials. What makes them
special is their numerical dominance, as well as the fact that they are
Are all pyramidal neurons the same? They have a strong family resemblance, with their upright posture of apical and basal dendrites; but they vary in their appearance across different species and cortical regions. Some may even merge with other types of excitatory neurons, like spiny stellate cells. Molecular approaches are revealing genetic diversity amongst pyramidal neurons, confirming the existence of distinct subtypes. Do different-shaped pyramidal neurons also function differently?
There is a lot of evidence for functional specialization by subtypes of
pyramidal neurons. Pyramidal neurons have long been a favorite of
neurophysiologists because they are big, bountiful and beautiful. Hence,
there is probably more known about the inner workings of these neurons
than any other class of neuron in the brain. For example, layer 5
pyramidal neurons are thought to benefit from their large size,
partitioning off parts of themselves into semi-autonomous processing
units. On the other hand, the smaller pyramidal neurons in the
hippocampus behave more like a committee, allowing all of their synapses
an equal
What happens when pyramidal neurons go wrong? As might be expected from their ubiquity and long axonal projections, faulty pyramidal cells are an important cause of brain disorders. Epilepsy, due to excessive neuronal excitation, is particularly common in brain regions containing many interconnected pyramidal neurons, such as the hippocampus. Some neurodegenerative disorders, including Alzheimer's disease, disproportionately kill pyramidal neurons, leading to characteristic cognitive changes. Thus, pyramidal neurons are the building blocks for high-level functions like memory and consciousness. When they misbehave, the consequences can be profound. |
The major light and electron microscopical features of neurons
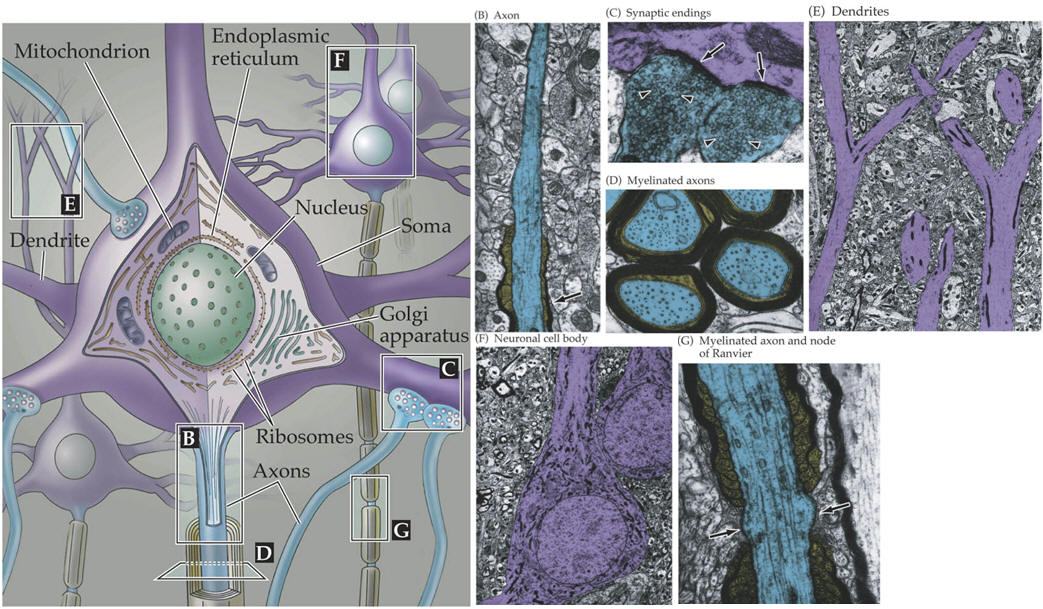
|
Synaptic plasticity Each neuron contains thousands of synapses. Dynamic changes in neuronal synaptic efficacy, termed synaptic plasticity, are thought to underlie information coding and storage in learning and memory. Synaptic plasticity can be regulated at the presynaptic side by altering the efficacy of neurotransmitter release, or on the postsynaptic side by changing the density, types, and properties of neurotransmitter receptors. AMPA receptors (AMPARs) are the principal ionotropic glutamate receptors that mediate fast excitatory synaptic transmission in mammalian brain. AMPARs are tetrameric assemblies of highly homologous subunits encoded by four different genes, GluA1-4 (see figure on the right). The life cycle of AMPARs from their biosynthesis, membrane trafficking, and synaptic targeting to their degradation are controlled by a series of orchestrated interactions with numerous intracellular regulatory proteins.
|
|
Structure of AMPAR. AMPAR is a tetrameric channel assembled from two dimers of different subunits, such as GluA1/GluA2 and GluA2/GluA3. Each individual subunit is composed of a large extracellular ligand-binding domain and a short intracellular carboxyl-tail linked by four membrane domains. Direct binding of AMPARs with C-terminal interacting proteins regulates various steps in AMPAR trafficking. |
|
One major mechanism that regulates synaptic strength involves the tightly regulated trafficking of AMPARs into and out of synapses. The trafficking of AMPARs into and out of synapses is highly dynamic and regulated by subunit-specific AMPAR interacting proteins as well as by various post-translational modifications that occur on their cytoplasmic carboxyl terminal (C-terminal) domains. Subunit composition of AMPARs thus determines the routes of AMPAR trafficking, such that GluA1 is dominant over GluA2 during activity-dependent AMPAR exocytosis, while GluA2 is the primary determinant during endocytosis and postendocytic endosomal sorting. This differential regulation is mainly due to interactions of intracellular C-terminal domain of GluA subunits with various components of the post-synaptic density (PSD) and their associated proteins that function during receptor internalization and exocytosis. The regulated trafficking of AMPARs is a major mechanism underlying activity-induced changes in synaptic transmission. In general, increases in AMPAR function at synapses result in the long-term potentiation (LTP) of synaptic strength, whereas removal of synaptic AMPARs leads to long-term depression (LTD). The number of AMPARs at synapses is dependent on relative rates of exocytosis and endocytosis at the postsynaptic membrane. Enhanced receptor exocytosis and recycling occur during synaptic potentiation, while increased rate of endocytosis results in LTD. The past few years have seen a rapid progress in the field revealing the complexity of AMPAR trafficking pathways (see figure below). |
|
|
|
| Routes of AMPAR trafficking. AMPARs are assembled in the endoplasmic reticulum and Golgi apparatus in the soma (and dendrites) and are delivered into the dendrite via kinesin-dependent vesicular trafficking on microtubule networks before their insertion to the plasma membrane. Surface AMPARs are incorporated into synapse and stabilized by postsynaptic density scaffolding proteins. Mature AMPARs undergo constitutive recycling through endosomal trafficking pathway. AMPARs are internalized from the plasma membrane by clathrin-mediated endocytosis and traffic to the early endosome. From early endosome, AMPARs can be delivered back to the plasma membrane either directly (fast recycling) or through recycling endosome, or entering the degradation pathway through late endosome. During LTD, the rate of AMPAR internalization outweighs the rate of AMPAR exocytosis, resulting in reduced number of synaptic AMPARs. Depending on the LTD stimulus, internalized AMPARs can either be retained in intracellular compartment or be degraded in lysosome. Conversely, during LTP, AMPARs are constantly delivered to the plasma membrane to induce early burst and long-term maintenance of synaptic potentiation. These highly complex pathways of AMPAR trafficking are tightly regulated by a series of orchestrated interactions with key intracellular regulatory molecules. Disruption of AMPAR binding to its interacting proteins shown in this diagram often leads to aberrant AMPAR trafficking, impaired synaptic plasticity and deficits in learning and memory. |
|
|
| Next page: Glia | Go back to: Cells |
|
|