|
MOLECULAR & CELLULAR
NEUROBIOLOGY
Master Course Cognitive Neuroscience - Radboud
University, Nijmegen
|
|
|
|
Chapter 5: Molecular biological research methodology |
| Bioinformatics - data analysis | CRISPR-cas genome editing |
| ChIP-chip/seq |
|
|
|
Gene transfer - transgenic animals |
|
Introduction (see also under "(Regulation of gene) transcription") |
|
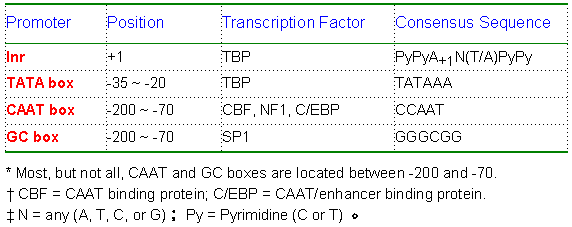
Gene transfer refers to the introduction of a DNA construct into a prokaryotic or eukaryotic cell. The promoter in the DNA construct is the region where the transcription initiation takes place. In prokaryotes, the sequence of a promoter is recognized by the Sigma (s) factor of the RNA polymerase. In eukaryotes, it is recognized by specific transcription factors. The most common promoter element in eukaryotic protein genes is the TATA box, located at -35 to -20 (see table below for other promoter elements). |
Eukaryotic promoter elements

Example:
|
Expression vectors
|
||||
|
To produce the protein encoded by a piece of cloned DNA, expression plasmids or vectors have been constructed (see Figure). To express the protein, the plasmid must be placed inside a cell (prokaryote or eukaryote). Once inside the cell, the promoter will attract the RNA polymerase and lead to the production of an mRNA. The cell's ribosomes will translate the RNA into a protein. This allows the production of large amounts of the protein, produce an epitope-tagged protein, or produce a range of protein concentrations if the promoter can be regulated. |
||||
|
|
||||
|
|
Map of a generic expression vector. This simplistic example contains an inducible promoter (red), your favorite gene (green), a gene that confers antibiotic resistance (blue), and an origin of replication (orange) used to produce many copies of the plasmid. |
|||
|
Similar to endogenous genes, expression vectors can contain DNA sequences that direct low level basal transcription, or more commonly, can have promoter elements linked to upstream regulatory sequences that serve as recognition sites for enhancer binding proteins (EBPs). There are four basic types of expression vectors used for modulate gene expression (see Figure): 1. Minimal promoters used to study gene regulatory elements such as enhancer elements; 2. Constitutive promoters used to direct expression of gene products; 3. Cell-specific promoters used to specify expression to target cells; 4. Regulated promoters used to control the on/off expression of cloned genes. Expression vectors contain DNA sequences flanking the multiple cloning site (MCS) that function as sequence-specific binding sites for transcription factors. Common DNA sequence motifs found in many types of promoters are represented by SP1, CAAT and AP1 binding sites. HREs are hormone response elements.
|
||||
|
|
||||
|
A popular way to regulate the amount and the timing of protein expression is to use an inducible promoter. An inducible promoter is not always active the way constitutive promoters are (e.g. viral promoters). Some inducible promoters are activated by physical means such as the heat shock promoter. Others are activated by a chemical such as IPTG or Tetracycline (Tet).
|
||||
|
Bacterial transformation, transfection, viruses and infection |
||||
|
Bacterial transformation is the process by which bacterial cells take up naked DNA molecules and incorporates them into its own genome. Click here for an animation on DNA transformation. Cell transfection: Introducing a cloned gene into eukaryotic cells (usually mammalian), such that it is taken up by the nucleus for the purpose of expressing a protein or RNA species. Transfection is one of several approaches to introduce cloned DNA into cells - the simplest and not the most powerful but can provide very valuable information quickly and with less preliminary work than other approaches. Conjugation is the bacterial version of sex. In conjugation, bacterial cells actually connect, and the "male" donates a piece of DNA to the "female." The piece of DNA in this case was excised earlier from the bacterial chromosome. Such excised pieces of DNA are called plasmids. Plasmids, being able to pass out of one cell and into another, are similar to viruses. But they have no protein coat and no "life cycle" different from that of their host cell; in this respect they resemble small chromosomes. Transduction is yet another way for bacteria to exchange genetic material. In transduction, a virus takes up a piece of DNA from its bacterial host and incorporates it into its own viral genome. After the virus has multiplied, many copies of the virus erupt from the infected cell. Depending on the kind of transduction, some or all of the daughter viruses take copies of parts of the bacterial DNA with them. When one of them infects a new cell, it inserts the stolen DNA into the new cell, where the stolen piece becomes integrated into the new cell's DNA. Transduction by viruses works in eukaryotic organisms as well. Viruses are mobile genetic elements Computer viruses are called viruses because they are analogous to biological viruses that infect living cells. A virus is a piece of genetic instructions, usually in a protective coat. Virus particles are tiny; a cell can manufacture and contain as many as a thousand of them before breaking open. They were first discovered when biologists observed that some disease-causing agents were able to pass through a filter too fine for bacteria. They can be small because they contain almost none of the machinery of a cell, only a smallish quantity of DNA (or RNA). Viruses are not living things. When they are outside of their host cell, they are just very complex molecular particles that have no metabolism and no way to reproduce. Having no independent metabolism they can remain viable indefinitely, under the right circumstances. Some of them can even be crystallized, like minerals. In this state they can survive for years unchanged — until they are wetted and placed into contact with their particular hosts.
The viruses that infect bacteria are more specifically called bacteriophages, or simply phages. The kind and amount of genetic instructions in phages vary from 3,600 RNA nucleotides to 166,000 DNA nucleotide pairs. The viruses that infect eukaryotic cells vary in size also. The poliovirus has 7,600 RNA nucleotides; the vaccinia (cowpox) virus has 240,000 DNA nucleotide pairs. When a virus attaches to its host cell, the host may take the whole virus into its cytoplasm where the virus's protective coat is removed. However, some bacteriophages use a different invasion method. They remain outside the cell and a chemical trigger causes them to inject their genome into the host's cytoplasm. Either way, the virus's genome enters the cytoplasm of the host cell. Once inside, the virus causes the machinery of the host cell to enter one of two cycles, the lytic cycle or the lysogenic cycle. In the lytic cycle, which leads to cell degradation, the host begins to carry out the reproductive instructions in the invading virus's genome. The host becomes a slave to the invader; it drops everything and begins to manufacture copies of the virus. After many copies have been made, the cell breaks open and dies, and many viruses are released. This is the normal way in which a virus causes symptoms of disease in its host. In the lysogenic cycle the host cell does not make more viruses, but simply harbors the entire viral genome in the cell, usually by incorporating it into the cell's genome. If the virus is an RNA virus, as many are, the RNA must first undergo "reverse transcription" into DNA. While harboring the viral genes, the cell may grow and multiply normally, carrying the new instructions harmlessly along with it. A virus carried in this manner is said to be latent. Sometimes after lysogenic integration of the viral genome into the host's DNA, an "induction event" can cause the viral infection to revert to the lytic cycle, in which the cell makes many copies of the virus and dies. After this happens, the numerous new virus particles can then infect many other cells. If the new infections are lysogenic, the virus's genes may again become integrated into the DNA of the new cells without harm to them. Lytic infection of one host followed by lysogenic infection in another is also called transduction. Eukaryotes are also known to acquire viral genes, and the phenomenon is not rare. Endogenous retroviruses and retroviral elements have been found in all vertebrates investigated. And it has now been shown that some of the genes that viruses install have a beneficial function for the host. In fact, viruses are now used to install genes for use in gene therapy. |
||||
Transgenic animals |
||||
|
|
||||
|
In genetically altered transgenic mice, an extra DNA sequence is added to the genome to :
The transgenes are usually developed from plasmids, and include a promoter region (which may be tissue-specific) and a coding sequence, usually a cDNA for the gene of interest. The plasmid is linearized and injected into the pronucleus of a fertilized oocyte (see below). This is then implanted into pseudopregnant females. Alternate approaches use minigenes derived from BACs or YACs (Bacterial- or Yeast Artificial Chromosomes). Transgenes are incorporated RANDOMLY into the genome, and are influenced by the position at which they integrate (position effect), a possible problem with transgenes. Position effects: presence of the endogenous gene; disruption of other genes.. |
||||
|
Microinjection of DNA Injection of linear DNA molecules into fertilized eggs (pronuclear stage) is performed with an inverted microscope, micromanipulation equipment and injection / holding devices. The first successful production of transgenic mice using pronuclear microinjection was reported in 1980.
|
|
|
||
|
With the ability to transfer or modify individual genes in an animal, we have a tremendous potential to understand the relationship between genes and development, and the causes of genetic diseases.We can also develop animal models for human diseases, such as cystic fibrosis, sickle cell anemia, and AIDS. "Pharming" is a term used to describe the production of human pharmaceuticals in animals. As an example, transgenic mice have been used to produce a human drug, tPA (tissue plasminogen activator to treat blood clots) since 1987. This type of approach for producing pharmaceuticals is particularly useful when the product is modified (glycosylated, etc.), and correct modification is not feasible in an in vitro system. A popular method of "pharming" is to couple the DNA gene for the protein drug with a DNA signal directing production in the mammary gland. The foreign protein is expressed and secreted into the milk (of a goat or cow, for example) and doesn't interfere with the health of the animal. A goat expressing tPA in its milk is worth an estimated $75,000 per year. A pig expressing human protein C (another clot-busting drug) is worth about $1,000,000 per year. Pig-napping may become a more common crime, as the pharming develops. |
|
|
||
|
|
||||
|
|
|||||||||||||||
|
Knock-out |
|||||||||||||||
|
With the ability to knock out (i.e. destroy) individual genes in an animal, we have a tremendous potential to understand the relationship between genes and development, and the causes of genetic diseases. In this approach, critical portions of the coding region of a given gene are removed so that no functional protein is produced. what the in vivo function of a gene is or one can develop a model of disease (only where the disease is a result of loss of function). In brief, the general procedure for generating gene-targeted knock-out mice is as follows. Embryonic stem (ES) cells heterozygous for a knockout mutation in a gene of interest (X) and homozygous for a marker gene (e.g. black coat color) are transplanted (see below) into the blastocoel cavity of 4.5-day embryos that are homozygous for an alternate marker (e.g. white coat color). The early embryos then are implanted into a pseudopregnant female. Some of the resulting progeny are chimeras, indicated by their black and white coats. Chimeric mice then are backcrossed to white mice; black progeny from this mating have ES-derived cells in their germ line. By isolating DNA from a small amount of tail tissue, it is possible to identify black mice heterozygous for the knockout allele. Intercrossing of these black mice produces individuals homozygous for the disrupted allele, that is, knockout mice. Some general problems with knock-outs are: lethality; developmental abnormalities; strain effects; altered expression of other genes. For conditional knock-out, see under "Conditional knock-out/knock-in in Neuroscience" (loxP/Cre system).
Knock-in
The knock-out mouse can be used to study only the effects of the loss of
a gene, not a specific mutation. For the latter purpose, a “knock-in”
method was developed, in which a mutated DNA sequence is exchanged for
the endogenous sequence without any other disruption of the gene. Some
knock-in strategies rely on the use of gene vectors with flanking
sequences (loxP), that on exposure to an enzyme (Cre recombinase)
undergo reciprocal recombination, leading to the deletion of the
intervening DNA. With this method, it is possible to replace a gene
sequence with a sequence of the investigator’s choice and to delete
unnecessary sequences (see figure below). The gene for Cre recombinase
has been knocked into targeted loci in a way that brings its expression
under the direction of the endogenous gene promoter, thus allowing
tissue-specific or temporalspecific expression of the Cre enzyme and
hence recombination of loxP sites that flank the gene of
interest. Applications of this method are numerous, and some are already
clinically useful. For example, knock-in of segments of the human
immunoglobulin gene into the mouse genome enables mice to produce
therapeutically useful humanized antibodies. As gene-targeting
technologies and strategies evolve, it may become possible to create
mouse models of polygenic human diseases such as diabetes and
hypertension.
Knockout and knock-in mice. A gene-targeting vector (left panel) is constructed to delete a specific exon of a gene in embryonic stem cells. Several kilobases of DNA on either side of the target gene are cloned around a drug-selection marker. After the cloned DNA (targeting vector) is introduced into the stem cells, positive and negative drug selection occurs in culture. The left panel shows a targeting vector that was constructed with loxP sequences flanking the positive drug-selection gene. Cre recombinase can delete the DNA sequence between the loxP sites, thereby deleting a specific gene in the embryonic stem cells. Knock-in mice (right panel) are generated by replacement of an endogenous exon with one harboring a mutation of interest. The gene-targeting strategy is similar to that used for knockout mice, except that a replacement exon (indicated by a star) is exchanged with the endogenous exon. Cre–loxP strategies can delete most traces of the targeting vector. Once the desired stem-cell clone is selected, it is injected into a blastocyst, which is implanted into the uterus of a foster mother. If the gene-targeted stem cells contribute to germ cells in the chimeric mice, subsequent offspring will harbor the gene-targeted mutation (germ-line transmission). |
|||||||||||||||
|
Conditional knock-out/knock-in The Cre/lox system for generating conditional knock-out mice (produce a mouse that no longer has a target gene in only one cell type) has been used as a way to artificially control gene expression. The system concerns a clever way to move pieces of DNA around in a cell (see figures below). Over the years, this system has allowed researchers to create a variety of genetically modified animals with the gene of their choice being externally regulated. This has contributed to our understanding of how individual genes and proteins work. Example: serotonin is implicated in mood regulation, and drugs acting via the serotonergic system are effective in treating anxiety and depression. Specifically, agonists of the serotonin-1A receptor have anxiolytic properties, and knockout mice lacking this receptor show increased anxiety-like behaviour. A tissue-specific, conditional rescue strategy was used to show that expression of the serotonin-1A receptor primarily in the hippocampus and cortex, but not in the raphe nuclei, is sufficient to rescue the behavioural phenotype of the knockout mice. Furthermore, using the conditional nature of these transgenic mice, receptor expression during the early postnatal period, but not in the adult, appeared to be necessary for this behavioural rescue. Thus, postnatal developmental processes help to establish adult anxiety-like behaviour.
|
|||||||||||||||
|
Knock-down In addition to the possibility of knocking out (i.e. destroy) or knocking in (i.e., bring in; e.g. humanize the mouse) a gene (see above), it has become possible to knock-down (i.e. reduce the expression of) individual genes in an animal, allowing functional and other studies. |
|||||||||||||||
|
Antisense RNA Messenger RNA (mRNA) is a single-stranded molecule that is used as the template for protein translation. It is possible for RNA to form duplexes, similar to DNA, with a second sequence of RNA complementary to the first strand. This second sequence is called antisense RNA (see figure). The formation of double stranded RNA can inhibit gene expression in many different organisms including plants, flies, worms and fungi. |
|
||||||||||||||
RNA interference (RNAi)
|
|
|
|
|
|
Figure: Mechanisms for siRNA- or miRNA-mediated gene silencing: 21–23 nt siRNAs (left) or miRNAs (right) are processed from long, bimolecular or intramolecular RNA duplexes by the enzyme Dicer and ATP. For miRNAs, only one strand appears to be stable whereas for siRNAs both strands can be detected at this stage. Subsequently, miRNAs and siRNAs assemble into RISCs (requiring ATP) that contain a single-stranded RNA molecule and target mRNAs either in their open-reading frames (ORFs) or 3’-untranslated regions (UTRs). For miRNAs this leads to translational arrest, whereas for siRNAs this results in cleavage of the mRNA target at the site of complementarity. As opposed to animal miRNAs, the majority of plant miRNAs have been proposed to function similarly to siRNAs, namely degradation of mRNAs. |
|
|
|
To view a video on RNA interference/antisense RNA, click on the picture.
|
|
| Next page: Optogenetics | Go back to: Generation of gene expression atlases of the central nervous system |
|
|