|
MOLECULAR & CELLULAR
NEUROBIOLOGY
Master Course Cognitive Neuroscience - Radboud
University, Nijmegen
|
|
|
|
Chapter 5: Molecular biological research methodology |
| Bioinformatics - data analysis | CRISPR-cas genome editing |
| ChIP-chip/seq |
|
|
|
CRISPR-cas9 genome editing
|
|
As we saw above and in other sections, technologies
for making and manipulating DNA have enabled advances in biology ever since the
discovery of the DNA double helix. Early approaches to introduce site-specific
modifications in the genomes of cells and organisms relied on the principle of
site-specific recognition of DNA sequences by oligonucleotides, small molecules,
self-splicing introns or site-directed nucleases (e.g. TALENs). The field of
biology is now experiencing a transformative phase with the advent of the
molecular toolbox for mammalian genome engineering: easily
programmable RNA-guided nucleases, which are derived from microbial adaptive
immune systems. Gene-editing technologies in the form of Clustered Regularly Interspaced Short Palindromic Repeat
(CRISPR)–CRISPR-associated protein (Cas) systems stand poised to enable
fast and accurate alterations of genomic information in mammalian model systems
and human tissues. The CRISPR-Cas9 technology originates from
type II CRISPR-Cas systems, which provide bacteria
with adaptive immunity to viruses and plasmids. After identification of the CRISPR/Cas9 system in
bacteria, a series of elegant biochemical studies distilled the essential
site-specific deoxyribonucleic acid cleavage activity to only two components: 1)
an RNA guide sequence and 2) a DNA endonuclease (see figure below). Based on
these observations, yet another group of investigators re-engineered these
components to function in mammalian cells, and it was discovered subsequently
that the CRISPR/Cas9 system could be manipulated to generate either random
mutations or targeted repair. Aside from cultured cells, the CRISPR/Cas9 system
functions efficiently to modify the genome of fertilized mouse zygotes, and
this approach has been adopted rapidly as an efficient method for creating
genetically altered mice.
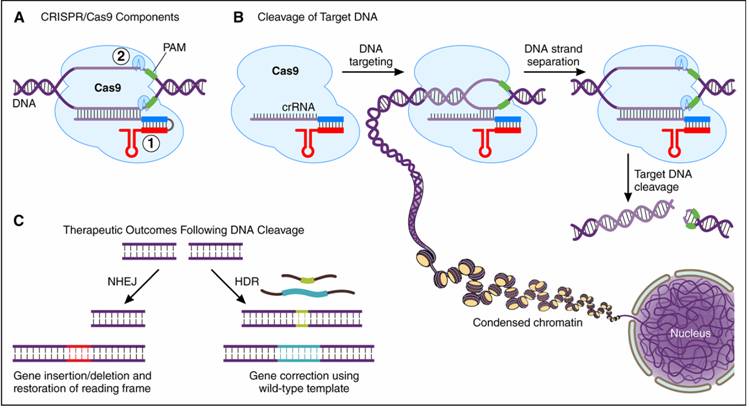
Figure: The CRISPR (Clustered Regularly
Interspaced Palindromic Repeat)/Cas9 system. A, The CRISPR/Cas9 system can be
distilled to two essential components: 1) a ribonucleic acid guide sequence to
target host deoxyribonucleic acid (DNA) containing a protospacer
adjacent motif (PAM) and 2) the Cas9 endonuclease enzyme. B, DNA cleavage
involves target recognition amid the complex genomic milieu followed by DNA
strand separation and precise enzymatic scission of the DNA backbone. C, After
DNA cleavage, nonhomologous end joining (NHEJ) can
potentially lead to restoration of an out-of-frame transcript without needing a
DNA template. Alternatively, the homology-directed repair (HDR) pathway can use
an error-free template to correct a gene mutation. Cas9 is an endonuclease that uses a guide
sequence within an RNA duplex to form base pairs with DNA target sequences,
enabling Cas9 to introduce a site-specific double-strand break in the DNA (see figure
below). The RNA duplex(tracrRNA:crRNA) was engineered
as a single guide RNA (sgRNA) that retains two
critical features: a sequence at the 5’ side that determines the DNA target site
by Watson-Crick base-pairing and a duplex RNA structure at the 3’ side that binds
to Cas9. This finding created a simple two-component system in which changes in
the guide sequence of the sgRNA program Cas9 to
target any DNA sequence of interest. The simplicity of CRISPR-Cas9 programming,
together with a unique DNA cleaving mechanism, the capacity for multiplexed
target recognition, and the existence of many natural type II CRISPR-Cas system variants, has enabled remarkable developments
using this cost-effective and easy-to-use technology to precisely and
efficiently target, edit, modify, regulate, and mark genomic loci of a wide
array of cells and organisms (see figures below). The power of the technology includes the systematic
analysis of gene functions in mammalian cells, study genomic rearrangements and
the progression of cancers or other diseases, and correct genetic mutations
responsible for inherited disorders. The
CRISPR/Cas9 system has thus evolved quickly from the initial discovery of an
adaptive immune system in bacteria to a 2-component genome editing tool to a
global disease-modification strategy. CRISPR/Cas9 technology has also revolutionized
how gene perturbation experiments are conducted at a genome-wide scale and has
enabled the unprecedented creation of genetically altered nonhuman primates for
preclinical studies. Eventual therapeutic translation, however, will require
identification of appropriate disease targets and development of robust methods
for introducing CRISPR/Cas9 components into specific cell-types without
off-cell-type and off-target effects. Despite these immediate hurdles,
CRISPR/Cas9 technology is poised to revolutionize disease management in ways
that were once difficult to imagine.
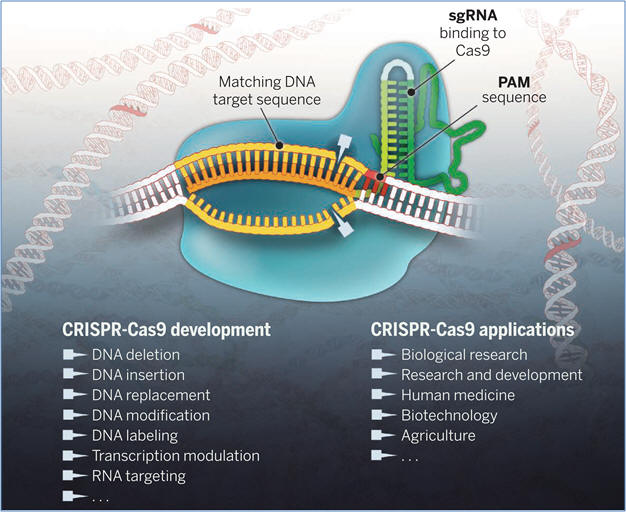
Figure: The Cas9 enzyme
(blue) generates breaks in double-stranded DNA by using its two catalytic
centers (blades) to cleave each strand of a DNA target site (gold) next to a PAM
sequence (red) and matching the 20-nucleotide sequence (orange) of the single guide
RNA (sgRNA). The sgRNA includes a dual-RNA sequence derived from CRISPR RNA (light
green) and a separate transcript (tracrRNA, dark
green) that binds and stabilizes the Cas9 protein. Cas9-sgRNA–mediated DNA
cleavage produces a blunt double-stranded break that triggers repair enzymes to
disrupt or replace DNA sequences at or near the cleavage site. Catalytically
inactive forms of Cas9 can also be used for programmable regulation of transcription and visualization of genomic loci.
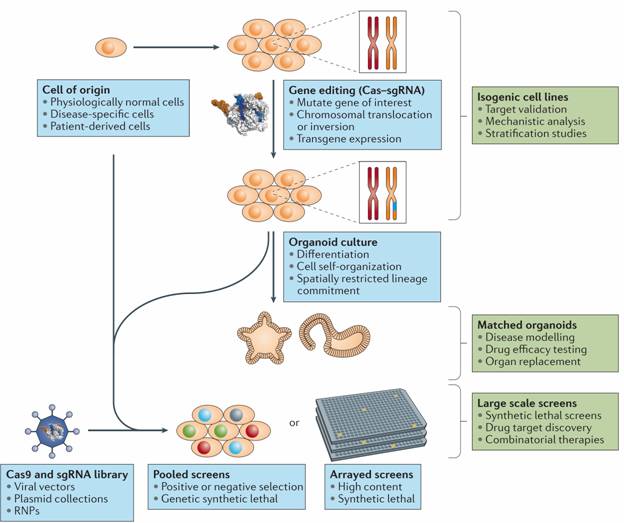
Figure: CRISPR–Cas in the generation of cellular models and large-scale screens. CRISPR–Cas gene editing can be used to generate isogenic cell lines for drug target validation, mechanistic analysis and patient stratification studies. Isogenic cell lines can also be used to generate organoids, which are particularly useful for modelling differentiation and self-organization processes. Large-scale single guide RNA (sgRNA) libraries can be used for high-throughput pooled or high-content arrayed screens, either in unmodified or in CRISPR–Cas-edited cell lines. RNPs, ribonucleoproteins.
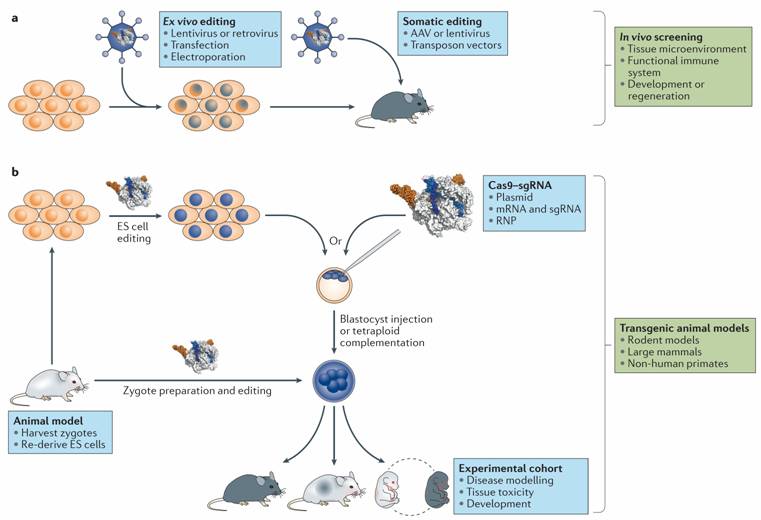
Figure: Applications
of CRISPR–Cas in in vivo screens and the
generation of animal models. a. Ex vivo editing
can be used to generate a library of modified cells for transplantation into
recipient animals. Alternatively, editing reagents can be delivered to host
animal tissues directly for somatic in situ editing. b.| CRISPR–Cas has also revolutionized the generation of transgenic
animal models through facile editing of embryonic stem (ES) cells for
traditional gene targeting and by enabling direct zygote editing in most
species. Zygote editing can be done ex vivo by electroporating
or microinjecting zygotes with CRISPR–Cas constructs
in the form of plasmids, RNA preparations or ribonucleoproteins (RNPs). AAV, adeno-associated virus; sgRNA,
single guide RNA.
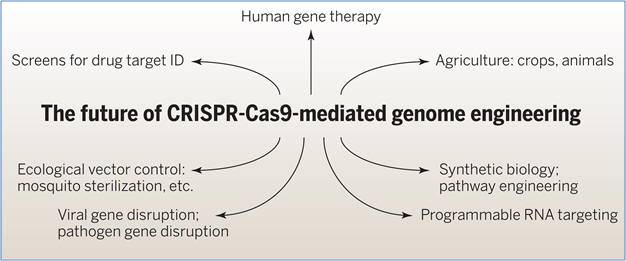
Figure: Applications in biomedicine and biotechnology.
Developments include establishment of screens for target identification, human
gene therapy by gene repair and gene disruption, gene disruption of viral
sequences, and programmable RNA targeting.
|
| Next page: Neurodevelopment | Go back to: Stem cells |