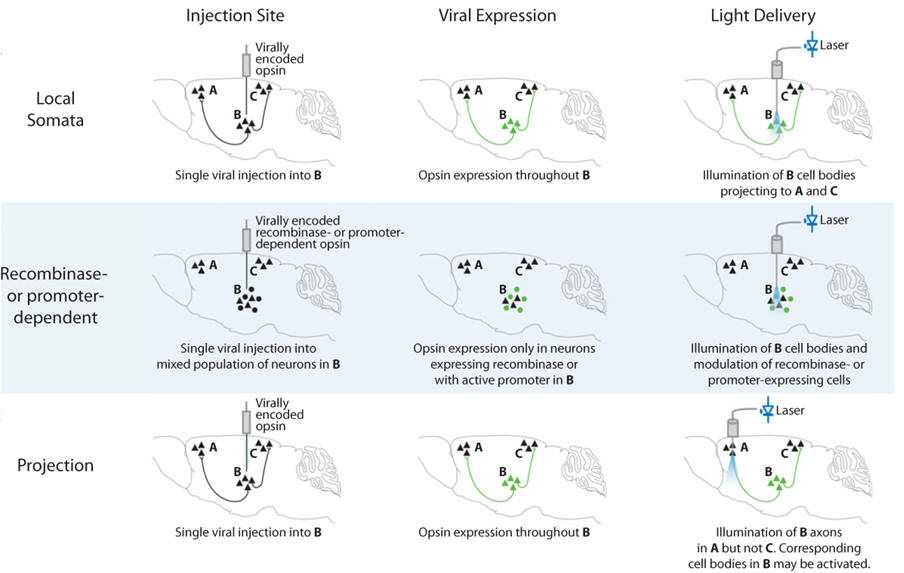
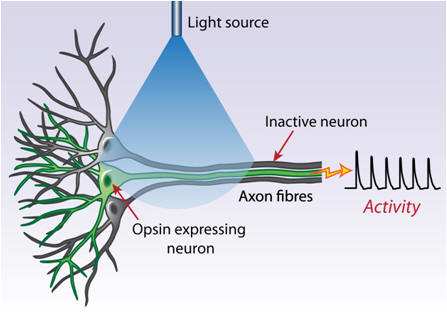
(2) Projection targeting. Cells could look the same from the genetic standpoint but serve fundamentally different functions by virtue of differential wiring. Projection targeting is the ability to selectively drive or inhibit cells defined by their wiring or projections. Microbial opsin gene products, especially with assistance from molecular engineering such as the addition of cellular trafficking motifs may traffic down dendrites or axons and create light-sensitive projections. This property, in the setting of anatomical specificity provided by viruses, allows transduction of cell bodies in one brain region and illumination of axonal projections in another (Figure 3C), thereby defining a cell population for excitation or inhibition by virtue of its connectivity. This approach provides a versatile promoter-independent means to control cells, requiring only anatomical information, and even with simple light guidance strategies this method can be applied to projections as short as hundreds of micrometers. A caveat of this approach is that all local photosensitive axons will be driven by light, even fibers of passage that do not synapse in the illuminated region.
(3) Delivering optogenetic tools into animals. Generation of mouse transgenic lines directly expressing opsin genes under local promoter-enhancer regions provides a distinctly useful means of achieving cell-type-specific opsin expression. While transgenic mouse lines require effort, time, and cost associated with their production, validation, and maintenance, the convenience and reliability of homogeneous opsin-expressing animals provides major experimental leverage. The use of transgenic or knock-in animals obviates viral payload limitations and thus allows for tighter control of transgene expression using larger promoter fragments or indeed the endogenous genome in full via knock-in.
(4) Spatiotemporal targeting. Cells may also be targeted by virtue of their birthdate or proliferation status, location at a moment in time, and other versions of what might be called spatiotemporal targeting; this approach has reached its most advanced state in the course of targeting specific neocortical layers. A long-sought goal of neuroscience has been to tease apart the role of specific layers, and of layer-specific neurons, in cortical microcircuit processing, brain-wide network dynamics, and animal behavior. In utero electroporation (IUE) may be employed to target opsins to distinct layers of the cortex, capitalizing on the sequential layer-by-layer ontogeny of neocortex in mammals, by incorporating the DNA into neurons generated during a specific embryonic stage. Beyond this special targeting capability, an additional unique advantage of IUE is that opsins are expressed from before the time of litter birth (allowing electrophysiological experiments at a younger stage than with viral expression). In principle, cells may also be targeted for optogenetic control by (i) active proliferation status at a particular moment in time, using cellcycle-dependent Moloney-type retroviruses; (ii) location at a particular moment in time (e.g., via migration through a particular anatomical location during development; and (iii) other methods including ex vivo sorting followed by transduction and transplantation. In general, the range of genetic techniques for delivering opsin genes into the brain has become broad and versatile.
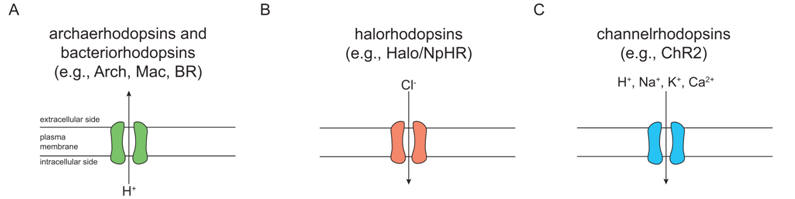
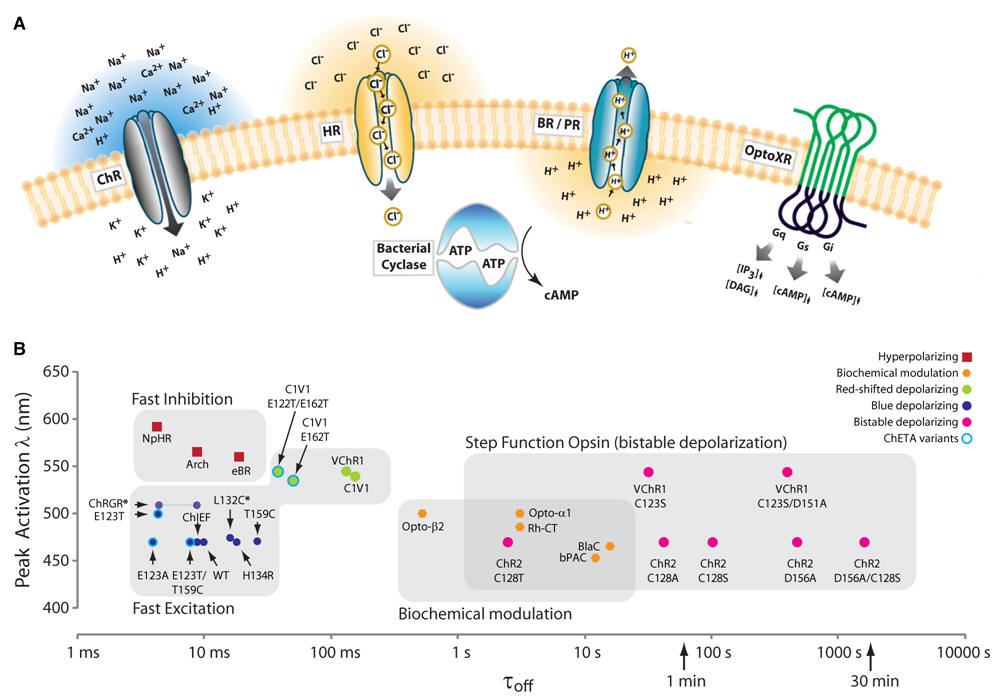
 4
kb), specific,
and strong may
be used, and
these are rare.
Several
potential different recombinase-dependent viral vector designs have
emerged and a Cre recombinase-dependent double-floxed inverted opsin
gene in AAV under the EF1a promoter was ultimately found to provide a
suitable combination of strength and specificity to enable behaviorally
significant optogenetic gain or loss of function within the constraints
of the freely moving mammal system. Not only is this strategy versatile
in the sense that it can be applied at will to the large and growing
pool of Cre driver lines, soon to include rat as well as mouse lines,
but this approach is also by design expandable along new dimensions that
enable combinatorial experiments (Figure
3).
4
kb), specific,
and strong may
be used, and
these are rare.
Several
potential different recombinase-dependent viral vector designs have
emerged and a Cre recombinase-dependent double-floxed inverted opsin
gene in AAV under the EF1a promoter was ultimately found to provide a
suitable combination of strength and specificity to enable behaviorally
significant optogenetic gain or loss of function within the constraints
of the freely moving mammal system. Not only is this strategy versatile
in the sense that it can be applied at will to the large and growing
pool of Cre driver lines, soon to include rat as well as mouse lines,
but this approach is also by design expandable along new dimensions that
enable combinatorial experiments (Figure
3).