Blood stem cells (Hematopoietics)

Embryonic stem cells
|
MOLECULAR & CELLULAR
NEUROBIOLOGY
Master Course Cognitive Neuroscience - Radboud
University, Nijmegen
|
|
|
|
Chapter 5: Molecular biological research methodology |
| Bioinformatics - data analysis | CRISPR-cas genome editing |
| ChIP-chip/seq |
|
|
Stem cells
|
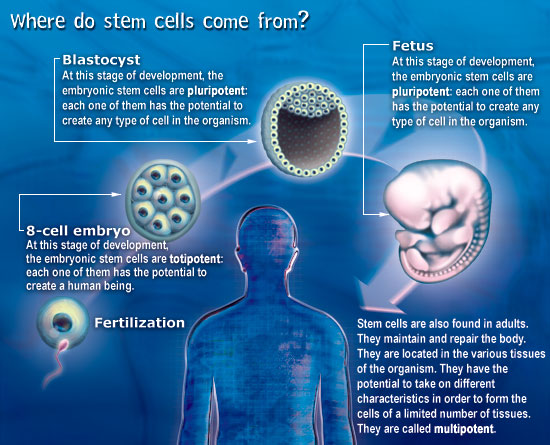
Stem cells are cells that have not yet made a career choice (see below for a more scientific definition). They can specialize to become any type of cell in the human body. Stem cells appear in the human about one week after conception. Scientists are very interested in them because they have so many different uses. Stem cells are capable of regenerating human tissues. One day, they may serve to treat all kinds of illnesses, repair tissues, and even create replacement organs. Already they are used to treat blood diseases such as leukemia. However, because of their origin, their use is very controversial. |
|
|
Blood stem cells (Hematopoietics) |
|
|
Embryonic stem cells |
|
New discoveries in the field of stem cells increasingly dominate the news and scientific literature. Wave upon wave of papers has led to an avalanche of new knowledge and research tools that may soon lead to new therapies for cancer, heart disease, neurological disorders, diabetes, and a wide variety of other diseases that afflict humanity. The history of science makes it certain that the knowledge derived from research on stem cells will eventually lead to enormous benefits for human health, even if they are unpredictable. Eventually, we will be able to use our profound understanding of biology to grow new organs that can be safely transplanted into human patients, and the work with stem cells will no doubt make important contributions to this breakthrough. As work continued, stem cells were isolated and characterized. Despite many studies, the expansion of human stem cells by in vitro culture proved to be difficult. Gene therapy of human stem cells remained an attractive but elusive goal. It had long been assumed that hematopoietic stem cells would produce only hematopoietic cells, but intriguing data began to suggest that marrow stem cells might generate other tissues such as a liver, a heart, or even a central nervous system. These and other tissues and organs seemed to have their own stem cells, and these stem cells might not be lineage specific. The plasticity of stem cells, or transdifferentiation, became a major subject of study. Techniques for obtaining stem cells from embryonic tissues were developed and seemed to offer even greater utility. Application of these stem cells to a variety of otherwise incurable human diseases became a possibility. |
Definition of a stem cell
|
Stem cells are defined functionally as cells that have the capacity to self-renew as well as the ability to generate differentiated cells. More explicitly, stem cells can generate daughter cells identical to their mother (self-renewal) as well as produce progeny with more restricted potential (differentiated cells). This simple and broad definition may be satisfactory for embryonic or fetal stem cells that do not perdure for the lifetime of an organism. But this definition breaks down in trying to discriminate between transient adult progenitor cells that have a reduced capacity for self-renewal and adult stem cells. It is therefore important when describing adult stem cells to further restrict this definition to cells that self-renew throughout the life span of the animal. Another parameter that should be considered is potency: Does the stem cell generate to multiple differentiated cell types (multipotent), or is it only capable of producing one type of differentiated cell (unipotent)? Thus, a more complete description of a stem cell includes a consideration of replication capacity, clonality, and potency, i.e. a working definition of a stem cell is a clonal, self-renewing entity that is multipotent and therefore can generate several differentiated cell types. The origin or lineage of stem cells is well understood for embryonic stem (ES) cells; the origin of stem cells in adults is less clear and in some cases controversial. It may be significant that ES cells originate before germ layer commitment, raising the intriguing possibility that this may be a mechanism for the development of multipotent stem cells, including some adult stem cells. In summary, stem cells—characterized by the ability to both self-renew and to generate differentiated functional cell types—have thus been derived from the embryo and from various sources of the postnatal animal. It is customary to classify stem cells according to their developmental potential (Table 1). In mammals, only the zygote and early blastomeres are totipotent and can generate the whole organism including extraembryonic tissues. Mouse ES cells are an example of pluripotent cells that can self-renew and generate all cell types of the body in vivo and in culture but are not able to generate the extraembryonic trophoblast lineage. Multipotent cells such as hematopoietic stem cells can give rise to all cell types within one particular lineage. Spermatogonial stem cells are an example of unipotent stem cells, as they can only form sperm. |
Stem cells of the early embryo
|
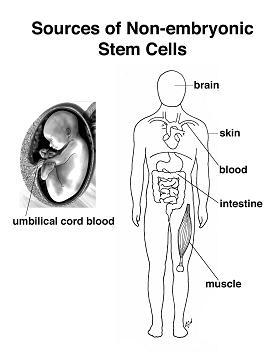
Biologists have explored the development of embryos of all sorts, from worms to humans, in search of the answer to the question of how a complex organism derives from a single cell, the fertilized egg. We now know many of the genes involved in regulating development in different species and find remarkable conservation of genetic pathways across evolution. We also have a good understanding of the logic of development—how the embryo repeatedly uses the same kinds of strategies to achieve cellular specialization, tissue patterning, and organogenesis. One common strategy of development is the use of the stem cell to help generate and maintain a given tissue or organ. As mentioned above, a stem cell is a cell that, when it divides, can produce a copy of itself as well as a differentiated cell progeny. During embryonic development cells stepwise lose their ability to form any kind of tissue. The cells gradually turn from totipotent cells (found in the morula stage of development) into pluripotent cells (such as found in the blastocyst stage) and, in almost all cases, into unipotent cells that can give rise to only one type of cell. During embryogenesis, cells are thus initially proliferative and pluripotent; they only gradually become restricted to different cell fates. The self-renewal capacity underlies the ability of adult stem cells, such as hematopoietic stem cells and spermatogonial stem cells, to constantly renew tissues that turn over rapidly in the adult. Even in tissues like the brain, where cells do not turn over so rapidly in the adult, there are long-lived quiescent stem cells that may be reactivated to repair damage. Much current research is focused on the identification, characterization, and isolation of stem cells from the adult, with the hope that such cells may be useful for therapeutic repair of adult tissues either by exogenous cell therapy or by reactivation of endogenous stem cells. However, to date, most adult stem cells have restricted potential, and achieving indefinite proliferation and expansion of the stem cells in culture is still not routine. |
Stem cells in the adult brain
|
The adult mammalian brain has vastly reduced regenerative potential compared to the developing brain. Nevertheless, cell proliferation occurs in the adult brain, as does neurogenesis, and cells that generate neurons and glia in vivo and in vitro have been identified in the adult CNS. As our understanding of the factors that regulate stem cell proliferation and neurogenesis in the adult brain deepens, the development of methods directed at repairing the damaged brain through the transplantation of cultured cells or through the induction of endogenous neurogenesis in selected neuronal populations may lead to novel therapeutic strategies. |
|
|
|
 |
Strategies of reprogramming somatic cells
Several different strategies, such as nuclear transplantation, cellular fusion, and culture-induced reprogramming, have been employed to induce the conversion of differentiated cells into an embryonic state (Figure 1). |
|
|
|
|
Figure 1. Four
strategies to induce reprogramming of somatic cells. (1) Nuclear
transfer involves the injection of a somatic nucleus into an enucleated
oocyte, which, upon transfer into a surrogate mother, can give rise to a
clone (“reproductive cloning”), or, upon explanation in culture, can
give rise to genetically matched embryonic stem (ES) cells (“somatic
cell nuclear transfer,” SCNT). (2) Cell fusion of somatic cells with ES
cells results in the generation of hybrids that show all features of
pluripotent ES cells. (3) Explantation of somatic cells in culture
selects for immortal cell lines that may be pluripotent or multipotent.
At present, spermatogonial stem cells are the only source of pluripotent
cells that can be derived from postnatal animals. (4) Transduction of
somatic cells with defined factors can initiate reprogramming to a
pluripotent state.
|
Reprogramming by defined transcription factors
|
In 2006, Takahashi and Yamanaka achieved a significant breakthrough in reprogramming somatic cells back to an ES-like state. They successfully reprogrammed mouse embryonic fibroblasts (MEFs) and adult fibroblasts to pluripotent ES-like cells after viral-mediated transduction of the four transcription factors Oct4, Sox2, c-myc, and Klf4 followed by selection for activation of the Oct4 target gene Fbx15 (Figure 2). |
|
|
|
|
Figure 2. Reprogramming of somatic cells to a pluripotent state. Transduction of the four transcription factors Oct4, Sox2, c-myc, and Klf4 into fibroblasts initiates the conversion to partially reprogrammed cells that express Fbx15 or to fully reprogrammed induced pluripotent stem cells (iPSCs) that express Oct4 or Nanog. The process involves a sequence of stochastic epigenetic events. |
Genetic-modification-free reprogramming to induced pluripotent stem cells (iPSCs)
|
Significant development in the reprogramming of somatic cells into induced pluripotent stem cells (iPSCs) has thus been achieved by retroviral infection of defined genes (Oct4, Sox2, Klf4, and c-Myc: “OSKC”; Figure 2). The efficiency with which iPSCs are generated is low, and little is known about the molecular mechanisms that govern their formation. However, recent advances have begun to shed light on the steps that occur inside the “black box” of reprogramming (Figure 3). The crucial molecular events that govern the transition from somatic cell to iPSC occur within 10–12 days of the initiating stimulus. Several recent studies have started to replace these genetic changes with specific chemical stimulation. Generation of iPSCs for use in the clinical setting would benefit from identification of alternative, ultimately safer, initiating stimuli, in preference to genetic modification. This could be transient treatment with defined factors, low-toxicity chemicals, or synthetic small molecules. An interesting advance in the efforts to replace retroviral expression of reprogramming genes has been recently described: conditioned media from MEFs overexpressing Wnt3a was also found to enhance the efficiency of OSK-iPSC generation by almost 20-fold. The soluble Wnt-induced effects were significantly impeded by the small molecule ICG-001. This molecule selectively represses Wnt/β-catenin-driven genes through the disruption of β-catenin and CBP (CREB-binding protein) interaction. The signaling pathway stimulated by Wnt3a could upregulate endogenous c-Myc that may lead to the epigenetic property of increased chromatin accessibility, required for pre-iPSC formation (Figure 3). Further advances in the discovery of chemicals and small molecules that promote epigenetic reprogramming, combined with forced activation of endogenous reprogramming genes, could realize the goal of iPSC generation from reprogramming-competent cells without the requirement for genetic manipulation. Greater understanding of the molecular mechanisms that mediate reprogramming will ultimately enable the production of safe human iPSCs and multipotential stem cells for use in clinical applications. |
|
|
|
|
Figure 3. Generation of iPSCs with fewer genetic modifications using chemical stimuli. Increase of chromatin accessibility by open chromatin formation through the Wnt- and c-Myc-mediated pathways or VPA treatment in pre-iPSCs. Reprogramming of adult neural stem cell (NSC) and fetal neural progenitor cell (NPC) to iPSCs by two genes (Oct4, Klf4) or the two genes plus one chemical (BIX-01294). AP, Alkaline phosphatase; MEF, mouse embryo fibroblast; CBP, CREB-binding protein; HDAC, histone deacetylase; VPA, valproic acid. |
iPSCs can provide models of human disease
|
The first use of iPSC technology might be the production of cell lines from patients with various diseases (Figure 4). For genetic diseases, iPSCs provide a new opportunity to analyse the pathways that lead to disease pathogenesis based on a particular genetic trait at the cellular level. A good example would be Huntington's disease, a neurodegenerative disease that is caused by CAG repeats in the gene that encodes huntingtin protein. The process that leads to cell death by expression of polyglutamine-containing molecules remains unclear, despite the presence of various cellular and animal models. Part of the difficulty is the absence of an appropriate human experimental system. If iPSCs from patients could be induced to develop into the relevant types of neuron, it would be the optimal study material for investigating the process of neural degeneration induced by these extra-long CAG repeats. The only other way to produce such cells would be by somatic cell nuclear transfer, a procedure that has not been successful in human cells. Neural cells from patients with Huntington's disease would be important tools for searching for drugs that reduce the toxicity of polyglutamine. iPSCs might prove to be even more useful in understanding the development of complex genetic disorders, such as type 1 diabetes and Parkinson's disease. In these diseases, it is difficult to analyse the pathogenic process in a prospective manner, as our current genetic knowledge is insufficient to predict who will develop the disease. However, if iPSCs could be derived from the somatic cells of such patients, and if immune cells or dopamine-producing neurons, respectively, could then be induced, it would represent a new way to prospectively investigate pathogenesis. iPSCs can be used to examine the effects of known genotypes on the cellular phenotype. An important possible application comes from the fact that iPSCs can be generated from any human who is taking a medicine. Thus, any effect or lack of effect of a particular drug that is detected during clinical treatment can be re-analysed using iPSCs from patients. This would greatly facilitate studies such as those to monitor drug safety once drugs are on the market. |
|
|
|
|
Figure 4. iPSCs as models for human disease. For many non-infectious complex diseases, it is difficult to predict who will develop a particular disease. Strategies to understand disease begin with the patient and include genetic analyses, epidemiology, statistics and studies in animal models. Induced pluripotent stem cell (iPSC) technology expands the opportunities for research. Patient-derived iPSCs can be used to examine the disease process at a cellular level. iPSCs can be used to test responses to possible drugs and might be used to develop patient-specific cell therapy. |
iPSCs for patient-specific therapy
|
Pluripotent cells offer great promise to the future of regenerative medicine and tissue engineering. As mentioned, to experimentally induce pluripotency in somatic cells (i.e. the ability of a cell to differentiate into any cell type of the three germ layers) nuclear transfer, direct reprogramming and cell fusion can be used and requires the activation of a transcriptional regulatory network. To date, no naturally occurring pluripotent cell has been identified in the mammalian soma, and cells with pluripotent potential in the early embryo or germ lineage are difficult to isolate from patients. This makes methods of experimentally induced pluripotency in readily available somatic cells (such as skin biopsies) invaluable for the generation of patient-specific stem cells. Pluripotent cells have also been shown to arise from ex vivo cultures of early embryonic cells and cells of the germ lineage, in which members of the pluripotency network are normally active, including cells of the morula, inner cell mass, epiblast, primordial germ cells and germ line stem cells. As mentioned above, iPSC technology has been successfully applied to human somatic cells, but the efficiency of derivation remains low. Nevertheless, human iPSCs might be an ideal cell source for cell therapy, given that iPSCs can be derived from the patient to be treated and thus are genetically identical cells that would avoid immune rejection. However, human iPSCs have not yet been directed to differentiate into a specific functional tissue or organ, although gene-expression profiles of in vitro differentiated human iPSCs have shown that they express different lineage markers. Furthermore, there are numerous challenges that remain before iPSCs might be considered for patient-specific therapy. The most desirable scenario would be to achieve high-efficiency reprogramming using only transient expression of the relevant factors and without using any oncogenes. Given the promise of patient-specific therapy, it might be prudent to consider the establishment of cell banks of iPSCs. These repositories would increase the number of patients who might be helped by iPSC-derived therapies. It will be necessary to characterize the developmental potential of human iPSC lines, and to compare the developmental potential of human ES and iPSCs. Human ES cells that have been grown in long-term culture have shown genetic instability, but the stability of iPSCs is yet to be investigated. In particular, the identical genetic and epigenetic profile of the iPS-derived cell type and the patient will need to be confirmed as cell production is scaled up. After the expansion and differentiation of iPSCs has been achieved, the resulting cells or tissues can be evaluated using the same regulatory criteria as those that have been developed from ES cells. These criteria include extensive documentation requirements to ensure, for instance, that no contamination by animal products has occurred during derivation and culture, and to prevent the introduction of potentially carcinogenic undifferentiated cells. Safety will be paramount for Phase I studies, and it will be necessary to show that differentiated cells that have developed from iPSCs will not develop into teratomas. The development and implementation of iPSC-based or ES-cell-based therapies will be far more complex than simple differentiation to replacement cells. Regulatory requirements will elongate the research timeline and increase the cost of such therapies. Patient-specific therapy is therefore unlikely to be widespread in the near future. The high costs for this therapy might mean it will never be applicable to large numbers of people. |
Human iPSCs to model a specific pathology
|
Spinal muscular atrophy (SMA) is an autosomal recessive genetic disorder and one of the most common inherited forms of neurological disease leading to infant mortality. An SMA characteristic is a selective loss of lower a-motor neurons resulting in muscle weakness, paralysis and often death. SMA is caused by mutations in the survival motor neuron 1 gene (SMN1) significantly reducing SMN protein expression and resulting in selective degeneration of lower a-motor neurons. A fascinating aspect of the disease is that a ubiquitous loss of SMN protein from all cells in the body results in the specific degeneration of the a-motor neurons. Model systems such as worms, flies or mice have provided invaluable data concerning the genetic cause of SMA, the mechanisms of motor neuron death and potential drug therapies. However, important limitations exist in these models (e.g. mice, flies and worms lack the SMN1-related SMN2 gene) and thus animal models require complicated knockout and overexpression strategies. Thus, a human cell-based assay system would be very beneficial. Until recently, SMA patient fibroblasts have been used to study SMA, but fibroblasts do not show the same vulnerability as motor neurons, and the processing and functioning of the SMN protein probably has unique features in a neural context that is highly relevant for understanding disease mechanisms. Also, motor neurons have a unique anatomy and physiology which may underlie their vulnerability to the disease process. Recent research resulted in the generation of iPSCs from skin fibroblast samples taken from a child with SMA (with his unaffected mother as control), showing for the first time that human iPSCs can be used to model the specific pathology seen in a genetically inherited disease (Figure 5). The generated iPSCs expanded robustly in culture, retained the capacity to generate differentiated neural tissue and motor neurons, maintained a lack of SMN1 expression, maintained the disease phenotype of selective motor neuron death and responded to compounds known to increase SMN protein. Overall, the iPSCs maintained the disease genotype and generated motor neurons that showed selective deficits compared to those derived from the child's unaffected mother. This means that human motor neurons carrying the SMA phenotype (appropriate morphology, specific markers and synapsin staining) were generated from a virtually limitless source of iPSCs. This discovery may help disease modelling (study disease mechanisms, recapitulating at least some aspects of SMA), e.g. to clarify further the role of SMN in disease initiation and progression, and provides additional strategies for tracking the differentiation of other subcellular structures in iPSC-derived motor neurons (that should help to determine why the disease is specific to motor neurons, with other types of neurons being unaffected). Furthermore, this breakthrough allows drug screening for SMA and the development of new therapies. Future iPSC technology could be used to better understand and develop treatments for several other genetically inherited diseases. |
|
|
|
|
Figure 5. Induction and differentiation process of human iPSCs to model spinal muscular atrophy (SMA). Following infection of the primary fibroblasts with lentiviral constructs encoding the pluripotency genes OCT4, SOX2, NANOG and LIN28, the generated iPC colonies were expanded and pushed toward the neural lineage (e.g. with retinoic acid, RA, and sonic hedgehog, SHH). The motor neurons derived from the SMA-iPSCs display a specific loss with longer time in culture.
|
|
|
|
|
|
Positron emission tomography scan. Left: normal brain - dopaminergic neurones are white/red. Right: Parkinsons brain, 10 years after transplant of human fetal dopamine neurons to the right striatum.Transplanted neurones survive and are functional.
Stem cells and cloning
|
|
|
| Next page: CRISPR-cas genome editing | Go back to: Cloning |
|
|