 FIG
1. Microbiology of a chromosome.
FIG
1. Microbiology of a chromosome.
Single-gene disorders
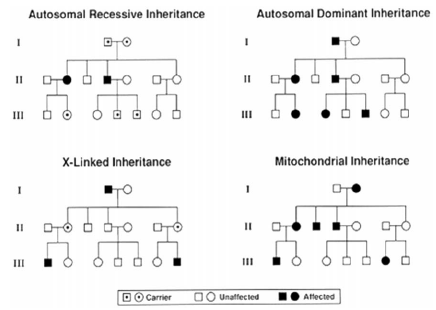
A single-gene disorder is an inherited disease caused by mutations in a single gene that are necessary and sufficient for the development of the phenotype. They show a Mendelian pattern of inheritance classified as autosomal-dominant, autosomal-recessive, or X-linked (dominant or recessive). Mitochondria has its own DNA which encodes for 37 genes. Diseases due to mitochondrial DNA mutations are only transmitted from the mother (no male-to-male transmission), since only ovum has mitochondria. It is important to realize that only a very small fraction of cardiovascular disorders are single-gene disorders and in the whole population the prevalence of single-gene disorders is rare, varying from 1 in 1000 to 1 in 10,000 and essentially never exceeds 1 in 500 (0.5%). It is estimated there are about 14,000 single-gene disorders, of which over 1500 of the genes have been identified. The genotype refers to the genetic basis, while phenotype refers to the observable features such as height, weight, or clinical features of a disease. One may possess the gene but not express the phenotype. The percentage of individuals with a gene that is expressed as a phenotype is referred to as penetrance and the variability in the clinical features of a particular expressed phenotype is termed expressivity. On average, a mutation occurs every 106 cell divisions or once every 200,000 years. Only mutations occurring in the gametes are transmitted. The patterns of inheritance are shown in the diagram in Fig 2. In autosomal-dominant disorders males and females are equally affected; an offspring of an affected parent will have a 50% chance of inheriting the mutant allele. In sporadic cases, the mutations occur de novo in one of the germ lines of the parents but by definition is absent in the somatic cells of parents. Autosomal-dominant inheritance usually has variable expressivity. The following features are characteristic of autosomal-dominant inheritances (Fig 2): (1) each affected individual has an affected parent unless the disease occurs due to a new mutation or there is low penetrance; (2) there is usually an equal split (50/50) of normal and affected offspring born to an affected individual; (3) normal children of an affected individual will have only normal offspring; (4) equal portions of males and females are affected; (5) both sexes are equally likely to transmit the abnormal allele to male and female offspring and male-to-male transmission occurs; and (6) vertical transmissions through successive generations occur. Two other characteristic features that help differentiate this type of inheritance for autosomal-recessive disorders are delayed age of onset and variable clinical expression. In autosomal-dominant, the phenotype is observed despite only one of the gene or alleles being affected. In autosomal-recessive inheritance, both alleles are affected, otherwise there is no phenotype. Males and females are equally affected. Clinical uniformity is more typical and disease onset generally occurs much earlier in life than in autosomal-dominant. Recessive disorders are more commonly diagnosed in childhood and, on average, only one in four children or 25% will be affected. The following are characteristic of autosomal-recessive disorders: (1) parents are clinically normal in alternate generations (genetically are heterozygous); (2) alternate generations are affected, with no vertical transmission; (3) both sexes are affected with equal frequency; (4) each offspring of heterozygous carriers has a 25% chance of being affected, a 50% chance of being an unaffected carrier, and a 25% chance of inheriting only normal alleles.