Excitatory amino acid transmitters account for most of the fast synaptic
transmission that occurs in the CNS.
The amino acid glutamic acid (or in fact its charged form glutamate) is the major excitatory amino acid transmitters.
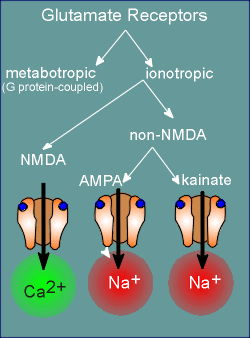 There are both ionotropic and metabotropic receptors for
glutamate.
There are both ionotropic and metabotropic receptors for
glutamate.
Among the ionotropic receptors we get a further breakdown into the so-called NMDA-receptors and the non-NMDA receptors.
NMDA stands for N-methyl-D-aspartate, an agonist for this class of receptors.
Many of the receptors of the NMDA family allows considerable Ca2+ to enter the cell and is thus considered to be a ligand-operated Ca2+ channel.
The non-NMDA receptors are, for the most part, Na+ channels.
There are two major families among the non-NMDA ionotropic receptors.
Again, they are named after the pharmacological agonist that has been used in initially defining the family.
One of the most important of the non-NMDA glutamate receptors is the AMPA receptors, with the chemical alpha-amino-3-hydroxy-5-methylisoxazoleproprionic acid as agonist.
The other family is named after the chemical agonist kainate.
All ionotropic glutamate receptors are constructed from subunits, with 5 subunits being required to make a receptor.
 From hydropathy profiles of
the various subunits it was originally thought that the general structure of the glutamate
ionotropic receptors is the same as the nicotinic receptors and GABAa receptors (i.e. 4
transmembrane regions with M2 forming the pore).
From hydropathy profiles of
the various subunits it was originally thought that the general structure of the glutamate
ionotropic receptors is the same as the nicotinic receptors and GABAa receptors (i.e. 4
transmembrane regions with M2 forming the pore).
Recent studies have questioned this assumption, however.
It is now believed that the second transmembrane of each subunit does not tranverse the entire membrane and thus the pore of glutamate receptors has a different structure from most other ligand-operated ion channels.
More information on this topic can be found by following the link to the models, given to the right.
The glutamate superfamily of iontropic receptors has the potential to produce an extremely large number of receptors.
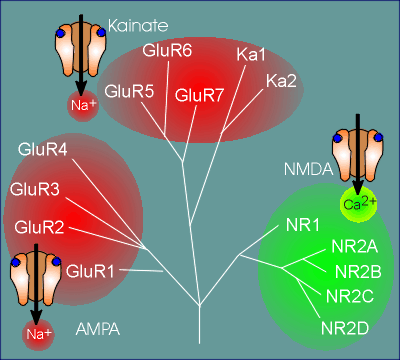 The first reason for this is that, within each family
group (i.e. NMDA, AMPA and kainate families), there are multiple genes coding for multiple
subunits from which to construct a receptor.
The first reason for this is that, within each family
group (i.e. NMDA, AMPA and kainate families), there are multiple genes coding for multiple
subunits from which to construct a receptor.
For example, for the NMDA family there are 5 genes which code for receptor subunits named NR1 (NMDA receptor 1) and NR2A, B, C, and D.
For the AMPA receptors there are 4 genes coding for subunits that have been called GluR1 to GluR4 (Glu = glutamate)
For the kainate family there are 5 genes, with some of the subunits being termed GluR and others Ka (Ka = kainate).
Among the AMPA and kainate receptors any subunit combination can make a functional receptor.
This has been shown by expressing the receptor subunits, either alone or in various combinations, in Xenopus oocytes, or other expression system such as certain tumor cells (review expression in Xenopus oocytes ?).
The electrophysiological properties of the expressed receptors are then studied to see if they are functional receptors.
In living systems only certain combinations of subunits are found.
A very active area of research is tracking down the subunit composition of the endogenous receptors in various brain areas.
more about the AMPA receptor
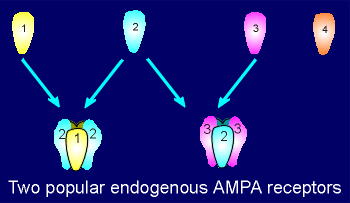 Among AMPA receptors the combination GluR1 with GluR2
is a very common, as is GluR2 combined with GluR3.
Among AMPA receptors the combination GluR1 with GluR2
is a very common, as is GluR2 combined with GluR3.
The GluR4 is not found very often in AMPA receptors from adult brain tissue but plays an important role in forming AMPA receptors during development.
More about the NMDA receptor
There are two special requirements in order to make a functional NMDA receptor.
First, the subunit NR1 must be included.
If no NR1 subunit is included then the receptor will not gate (open) when activated with NMDA or glutamate.
NMDA receptors usually have 2 NR1 subunits within the receptor, with the remaining three subunits being selected among the NR2 group of subunits.
The second requirement is that the NMDA receptor must have some NR2 subunits within it.
The reason for this is that only NR2 subunits contain a binding site for glutamate.
Thus, if there is no NR2 then there is no glutamate binding and thus there is no receptor activation.
In the NR1 subunit the homologous region to the glutamate binding site in fact forms a binding site, not for glutamte but for a co-agonist for the receptor, namely the amino acid glycine.
This may sound surprising because glycine itself is an inhibitory neurotransmitter with its own receptor, sturctrually similar to the GABAa receptor.
 Nonetheless, this transmitter is also required for
NMDA receptor function.
Nonetheless, this transmitter is also required for
NMDA receptor function.
Normally there is sufficient glycine in the extracellular compartment of the brain for NMDA receptor function and thus it is the presence or absence of glutamate which operates the receptor.
Use the link to left learn more about the structure and function of the NMDA receptor.
+++++++++++++
Besides the repertoire of subunits to select from in constucting a receptor, there are three other reasons that glutamate ionotropic receptors can have extremely diverse structures.
These are:
(1) alternative splicing of the primary transcript (precursor mRNA) to make mRNA
(2) post-transcriptional editing of the mRNA involving specific base substitutions
(3) post-translational modification of the receptor proteins, such as glycosylations whereby carbohydrates are attached to the protein.
As an example of alternative splicing, consider the NMDA NR1 subunit.
The gene coding for NR1 contains 22 exons and alternative splicing involving exons 5, 21 and 22 lead to the production of 8 different splice varients!
As an example of post-transcriptional editing, consider the AMPA Glu2 subunit.
Within the mRNA coding for Glu2 there occurs a site-specific mRNA editing whereby the codon coding for the amino acid glutamine is switched to arginine.
A receptor constructed with the glutamine variant (which occurs developmentally) displays considerably Ca2+ permeability whereas the arginine variant is clearly a Na+ channel.
+++++++++++++
Among the glutamate ionotropic receptors a distinction is made between the NMDA receptors and non-NMDA receptors because the NMDA receptor possesses two additional features, besides having a co-agonist, which makes it quite unique.
First, as mentioned above, the NMDA receptor is a ligand-operated Ca2+ channel.
Secondly, to be induced to open the NMDA receptor requires not only a ligand (glutamate) but also a simultaneous, or near simultaneous, depolarization of the membrane.
The consequences of these two unique features are discussed in further detail below.
Calcium has a unique feature, when compared to Na+, K+ and Cl- ions, in that it has the ability to bind-to many proteins.
This feature has been utilized in the use of Ca2+ as an intracellular message molecule.
 The most important Ca2+-binding protein in this regard is
calmodulin.
The most important Ca2+-binding protein in this regard is
calmodulin.
Calmodulin is present at high concentrations in the cytosol of every cell (i.e. it is ubiquitous).
Calmodulin may have started out as one of the many Ca2+ binding proteins in the cytosol which function as Ca2+ buffering proteins.
Use the link to the left for further details on the evolution of Ca2+ and calmodulin as important components of intracellular signaling processes.
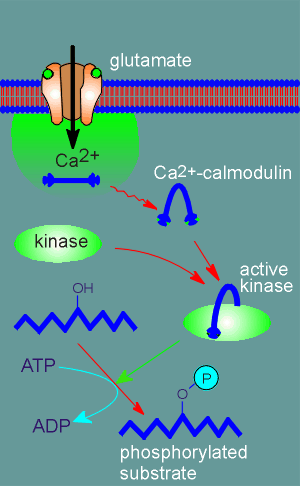 Calmodulin has a special feature, it changes shape when it binds Ca2+.
Calmodulin has a special feature, it changes shape when it binds Ca2+.
The calmodulin within the Ca2+ microdomain around an activated NMDA receptor binds Ca2+ (4 binding sites) and consequently changes shape.
It goes from a straight rod shape to the curve shape upon binding Ca2+.
The activated calmodulin (i.e. Ca2+ bound form) is free to diffuse out of the microdomain.
Also present in the cytosol are proteins that can bind to calmodulin when it is in its Ca2+ bound form.
One of the proteins that the Ca2+-calmodulin can bind to is a kinase, the so-called Ca2+-calmodulin-dependent protein kinase, often abbreviated CaMK.
The Ca2+-calmodulin straddles the kinase like a horseback rider (see illustration to right).
Kinases are enzymes that catalyze the attachment of a phosphate group from ATP to protein substrates.
In the Ca2+-calmodulin bound form the kinase is active and can thus it can phosphorylate substrate proteins within the cell.
This means that more permanent changes are brought about in the cell than simply a membrane depolarization, which is quickly reversed.
To reverse a phosphorylation takes time because a whole new set of enzymes, the phosphatases, must be activated to reverse the process.
Thus, among ion channel receptors the NMDA receptor is unique and powerful...because it allows considerable amounts of Ca2+ into the cell, it can bring about more permanent changes involving protein phosphorylations.
The NMDA receptor also allows considerable Na+ to enter through the channel, in fact Na+ is the major ion to pass through the NMDA receptor.
Nonetheless, the receptor allows sufficient Ca2+ to enter to activte Ca2+ dependent processes in the cell, and thus it is often referred to as a ligand-operated Ca2+ channel.
__________
Note: Calmodulin, and the kinase it activates, will be considered in more detail in the G protein coupled receptor section of these tutorials. Use this link if you would like to skip ahead to a movie showing a kinase phosphoryling a protein, use this link if you would like to skip ahead for more information on calmodulin and use this link if you would like to skip ahead to a movie showing calmodulin activating its kinase.
The second unique feature of a NMDA receptor is that it requires both ligand (glutamate) and a depolarization before it can be activated.
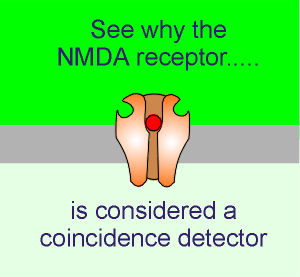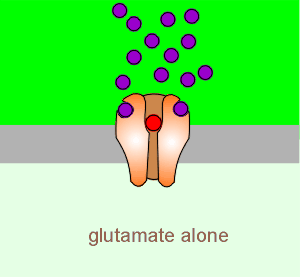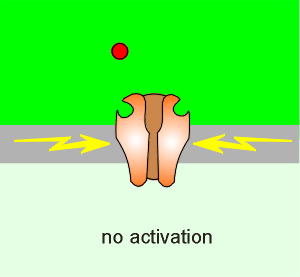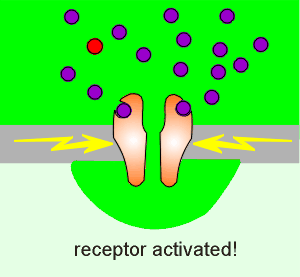 There is, within the pore of the NMDA receptor, a Mg2+ block (red ball in
figure to left).
There is, within the pore of the NMDA receptor, a Mg2+ block (red ball in
figure to left).
The membrane depolarization relieves this block, the Mg2+ comes out, and the receptor can activate (if there is glutamate present).
So...two events must occur for the NMDA receptor to be activated: There must be (1) a membrane depolarization to relieve the Mg2+ block and (2) there must be presynaptic release of the ligand glutamate.
For this reason NMDA receptors are considered coincidence detectors, i.e. they detect the coincidence of membrane depolarization and release of presynaptic glutamate.
It is only when those two events occur at the same time (i.e. in coincidence) that we have activation of the NMDA receptor.
Coincidence detection is thought to be important in learning processes and, indeed, the NMDA receptor has been found to be an essential component of the learning and memory processes in the vertebrate brain.
The fact that the NMDA receptor requires both ligand activation and membrane depolarization to become activated means that the receptor can act as an integrating machine (i.e. under conditions of where ligand is present plus there is a strong depolarization, the receptor creates a signal; when only one of these conditions have been meet it does not create a signal).
 The best studied NMDA receptors are found in the hippocampal
CA1 pyramidal cells.
The best studied NMDA receptors are found in the hippocampal
CA1 pyramidal cells.
Use the link to the left to take a closer look at the rat hippocampus and the receptors involved in the transmission of information through the CA1 region.
Researcher believe there are two ways that NMDA receptors can become activated in the synapses of the CA1 region.
The first is where the NMDA uses its coincidence detection properties to act as a signal strength discriminator.
To illustrate this consider the case where there is very low presynaptic activity (i.e. a very low amount of glutamate is released from the presynaptic terminals of the Schaffer collaterals.
Under these conditions a few non-NMDA receptors, the so-called AMPA receptors, are activated to give membrane depolarizations and EPSP.
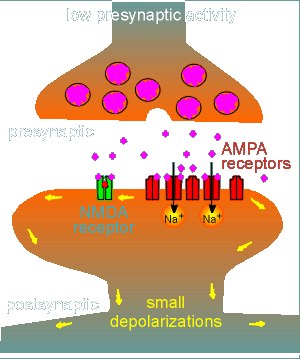 These depolarizations are brought about because the AMPA receptors are Na+
channels.
These depolarizations are brought about because the AMPA receptors are Na+
channels.
At low levels of activation (i.e. low level of glutamate release from the presynaptic neuron) the depolarization is insufficient to lift the Mg2+ block of the NMDA receptor.
Thus the NMDA receptor remains inactive despite the fact that glutamate (purple balls) has bound to the receptor.
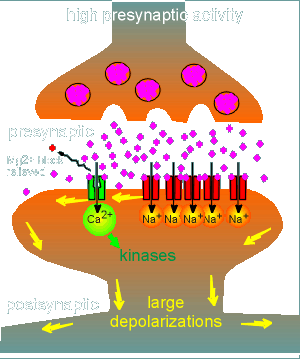 At a certain level of presynaptic activation (lets call it
threshold level), sufficient glutamate is released to activate sufficient non-NMDA
receptors to produce a sufficiently large depolarization to relieve the Mg2+ block of the
NMDA receptor.
At a certain level of presynaptic activation (lets call it
threshold level), sufficient glutamate is released to activate sufficient non-NMDA
receptors to produce a sufficiently large depolarization to relieve the Mg2+ block of the
NMDA receptor.
At this point the NMDA receptor becomes activated because the two criteria for receptor activation have been met (strong depolarization plus the presence of glutamate)
In this way the NMDA receptor has acted as a signal strength discriminator: at low levels of presynaptic activity it is inactive and, at threshold, it activates.
With activation of the NMDA receptor there is an influx of Ca2+, there is activation of kinases in the terminal and consequently long-lasting changes are made.
Recent studies have shown a second way the NMDA receptors can become activated.
This second mechanisms depends on the fact that when a neuron fires off an action potential, the action potential goes not only along the axon to the nerve terminal but also goes in the opposite direction through the cell body to the dendrites and then up the dendrites.
This is referred to as "back propagation" of the action potential (back propagation will be considered further in the next module of this tutorial).
Consider a synapse that has just been "lightly" activated by presynaptic release of glutamate and has just fired-off an action potential (perhaps through the action of other synapes in the dendritic field).
We now have a situation where both criteria for activation of the NMDA receptor can be meet...glutamate is present (from presynaptic release) and strong depolarization is present (from the back-propagated action potential).
The NMDA receptor is now acting as a true coincidence detector...the coincidence between (1) the fact that the neuron has just been activated to fire off an action potential and, (2) the fact that the synapse in question has just been activated to release glutamate.
Both mechanisms described above result in the activation of the NMDA receptor and thus the presence of a Ca2+ signal in the postsynaptic spine.
The Ca2+ can then activate kinases and the resulting phosphorylations can cause strengthening of the synapse so that the synapse makes a stronger response to glutamate.
This is an example of synaptic plasticity (the ability of synapses to change strength), a process important for learning and memory.
" Mechanisms of learning and memory" is one of the major topics in the lecture series of the course "Neurobiology"
There is good evidence to indicate that the AMPA receptor is responsible for maintenance of the synapses.
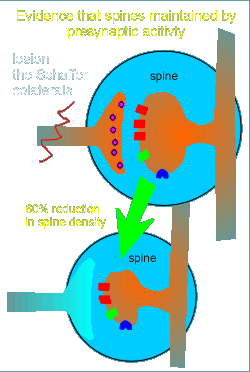 What is the evidence?
What is the evidence?
First, presynaptic stimulation is required....if Schaffer colaterals are lesioned (cut) then the density of the postsynaptic spines are reduced by 60% (depicted in figure to the right).
From this observation the idea was developed that glutamate might stimulate maintenance of the postsynaptic spine.
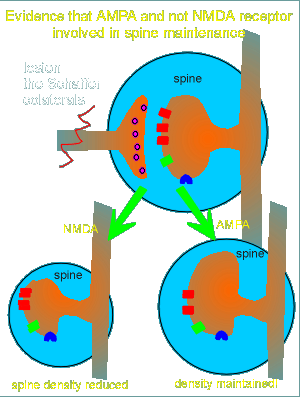 This idea is supported by the observation that AMPA can
overcome the effects of the lesion (figure to left).
This idea is supported by the observation that AMPA can
overcome the effects of the lesion (figure to left).
Apparently the AMPA receptor is solely responsible for maintenance of the postsynaptic spine.
This could be shown in seperate experiments where NMDA treatment was given.
In lesioned tissue NMDA treatment was unable to maintain the spine density (figure to left).
Further evidence that the AMPA and not the NMDA receptor is responsible for spine maintenance comes from studies with receptor antagonists (blockers).
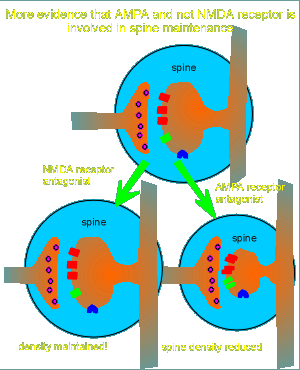 NMDA receptor antagonists had no effect on the spine (figure
to right).
NMDA receptor antagonists had no effect on the spine (figure
to right).
Treatment with AMPA receptor antagonists, however, caused a dramatic reduction in postsynaptic spine density.
From these and other studies it was concluded that glutamate, acting through AMPA receptors, is responsible for maintenance of the spine.
The idea is that the AMPA receptor is responsible for spine maintenance and also for signal transduction under conditions of low presynaptic activity.
The NMDA receptor is called into play only under conditions of high presynaptic activity as discussed earlier.
The postsynaptic mechanism of AMPA receptor maintenance of the spine is completely unknown.
At the presynaptic side the authors suggest (and present some evidence) that a low spontaneous release of glutamate is involved in spine maintenance.
It has been know for many years that neurons are never completely silent ..... they fire off an action potential every so often that has been described as "background noise".
The above studies suggest that perhaps this "background noise" has an important function, namely the maintenance of synapses.
 This is the end of the module "Glutamate Receptors"
This is the end of the module "Glutamate Receptors"