 The
hydrophobicity profiles of the glutamate ionotropic receptors are very similar to those
displayed by the nicotinic receptor and the GABAa receptor.
The
hydrophobicity profiles of the glutamate ionotropic receptors are very similar to those
displayed by the nicotinic receptor and the GABAa receptor. The
hydrophobicity profiles of the glutamate ionotropic receptors are very similar to those
displayed by the nicotinic receptor and the GABAa receptor.
The
hydrophobicity profiles of the glutamate ionotropic receptors are very similar to those
displayed by the nicotinic receptor and the GABAa receptor.
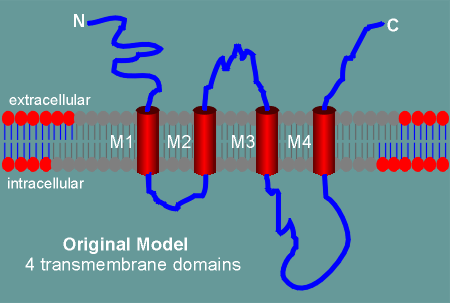
Thus the original model of the glutamate receptors had 4 transmembrane regions (M1 to M4).
As with the nicotinic and GABAa receptor, the M2 transmembrane domain was believed to form the ion pore.
This idea has been challenged by a number of recent molecular and biochemical studies (for example, immunocytochemistry at thelevel of the electron microscope, shows the C terminal of the subunits to be intracellular and not extracellular, as the model predicts).
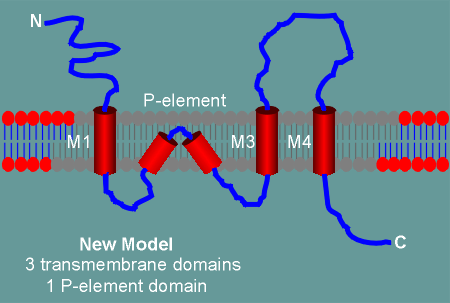 In the new model, for which there is now considerable experimental
evidence, the M2 domain does not completely traverse the membrane.
In the new model, for which there is now considerable experimental
evidence, the M2 domain does not completely traverse the membrane.
Rather, it forms a "kink" within the membrane and enters back into the cytoplasm.
The kinked M2 still forms the ion pore and thus it has been designated as the P-element (P for pore).
Thus with this new construction the C-terminal comes to lie inside the cell.
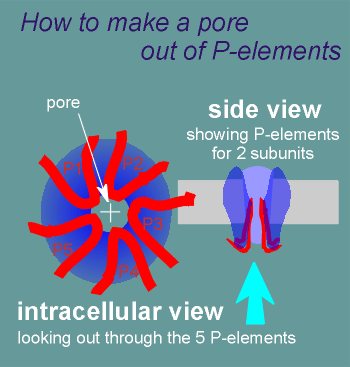 The P-elements form loops that push into the lipid bilayer creating the pore.
The P-elements form loops that push into the lipid bilayer creating the pore.
The pore contains serines and threonines and thus it is a hydrophilic pore similar to the nicotinic and GABAa receptor pores.
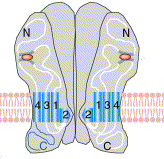 Shown to the left is another way of
visualizing a glutamte ionotropic receptor.
Shown to the left is another way of
visualizing a glutamte ionotropic receptor.
The orange oval shows the glutamate binding site.
Also shown are the M1, 3 and 4 regions for two of the subunits, with the M2 involved in forming the pore.
______________
The construction of a pore through P-elements is believed to be the way pores are constructed in voltage-gated ion channels as well (click here to skip ahead and see the structure of voltage-gated ion channels?)