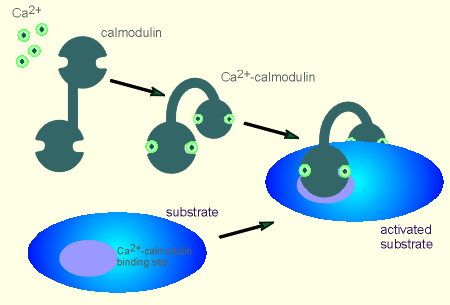
 When four Ca2+ ions
bind to calmodulin the entire structure of the protein changes.
When four Ca2+ ions
bind to calmodulin the entire structure of the protein changes.Calmodulin is a ubiquitous protein (i.e. it is found in every cell).
In fact, when it was discovered it was thought likely that this protein would have a structural function because it was present in every cell type and at high concentrations.
It was only much later found to have important signaling functions.
The protein has four binding sites for Ca2+, two in the N-terminal region and two in the C-terminal region.
It probably started life as a Ca2+ buffering protein (to protect the cell from high Ca2+ levels) and later acquired its important signaling properties.
Between Ca2+ binding domains in calmodulin is an alpha helix region.
 When four Ca2+ ions
bind to calmodulin the entire structure of the protein changes.
When four Ca2+ ions
bind to calmodulin the entire structure of the protein changes.
This can be seen in the illustration to the left.
It is now in a position to straddle its substrate by binding to two Ca2+-calmodulin binding sites.
Upon binding, the Ca2+-calmodulin induces structural changes in the substrate.
In the case where the substrate is a kinase, it activates the kinase activity.