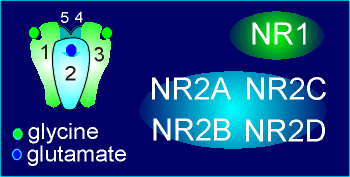
 The NMDA receptor is composed NR1 and NR2 subunits.
The NMDA receptor is composed NR1 and NR2 subunits. The NMDA receptor is composed NR1 and NR2 subunits.
The NMDA receptor is composed NR1 and NR2 subunits.
It must have at least one, and generally has two, NR1 subunits.
The NR1 subunit contains the binding site for glycine.
The NR2 subunits contains the glutamate binding site.
The glycine binding site in NR1 has been found to be homologous with the glutamate binding sites in the NR2 subunits.
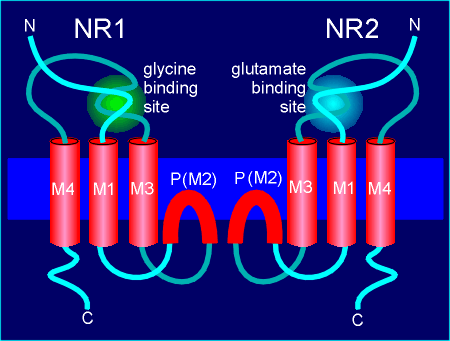 A more detailed representation of the NR1 and NR2 subunits, showing
these homologous sites, are given to the left.
A more detailed representation of the NR1 and NR2 subunits, showing
these homologous sites, are given to the left.
In this model the originally proposed M2 transmembrane region is shown to form the P loop which contribute to formation of the pore.
Glycine and glutamate are co-agonists of the NMDA receptor.
In order to be activated the receptor must bind both glycine and glutamate.
For receptor activation, glutamate is release from the presynaptic side of the synapse.
The concentration of glycine in the cerebral spinal fluid (and thus in the extracellular space) has been considered high enough to satisfy the NMDA receptors requirement for this agonist.
Thus the receptor channel is gated by glutamate binding to the receptor.
There is some recent evidence (to be discussed in the lectures) that the glycine concentration in the region of some glutamate synapses may be sub-optimal for receptor function.
Thus glycine, and mechanisms that regulate extracellular glycine concentrations, would participate in regulating NMDA receptor activity.