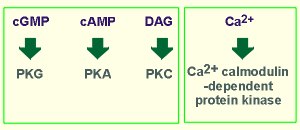
 There are four kinases regulated by second
messengers.
There are four kinases regulated by second
messengers. There are four kinases regulated by second
messengers.
There are four kinases regulated by second
messengers.
Their names reflect the second messenger which activates them.
Thus we have: (i) protein kinase G (PKG), activated by cyclic GMP, (ii) protein kinase A (PKA) , activated by cyclic AMP, (iii) protein kinase C (PKC), named for the first activator discovered for this kinase, namely Ca2+, and (iv) a family of kinases activated by Ca2+ -calmodulin, thus known as Ca2+-calmodulin-dependent protein kinases, often abbreviated CaMK.
PKG, PKA and PKC have a very similar structure and are thus considered to represent a family of kinases.
The Ca2+ calmodulin-dependent protein kinases have a different structure but do show some sturctural similarities to the above family.
In considering the kinases, I will begin with PKG, then consider PKA and PKC and, finally, the Ca2+ calmodulin-dependent protein kinases.
Movies of each of the kinases will be presented.
These movies do not show the actual phosphorylation from ATP, which was covered earlier in our consideration of tyrosine kinases.
To remind you, to the left is the movie that was presented earlier, showing the transfer of the phospho-group from ATP to a protein during a protein phosphorylation.
Keep in mind in the movies below that each phosphorylation shown involves the hydrolysis of an ATP to an ADP, as illustrated to the left.
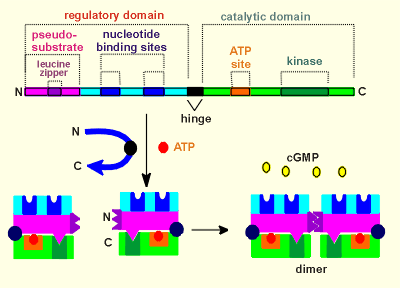 PKG has a regulatory domain in the N-terminal,
which possess the sites for cGMP binding (nucleotide binding sites).
PKG has a regulatory domain in the N-terminal,
which possess the sites for cGMP binding (nucleotide binding sites).
The catalytic domain is in the C-terminal (possessing the kinase itself and an site for ATP, which is the phosphate donor during a phosphorylation).
To make the kinase, the protein is folded around a hinge domain (see blue arrow), such that the N- and C-terminal come close together.
Now the so-called pseudo-substrate can fit into the kinase domain (thus blocking the kinase domain).
The internal foldings of the protein are such that the nucleotide binding sites come to lie in exposed positions for easy access to the cGMP.
The N-terminal region also contains a structure called a leucine zipper where proteins can bind to each other.
In the case of PKG two identical subunits are joined, via the leucine zipper domains, to form a dimer.
The complete PKG is a dimer, with four cGMP binding sites and two kinase domains.
To activate the kinase four cGMP molecules must bind to the nucleotide binding sites.
Shown to the right is a move of the activation of PKG.
Notice how the pseudo-substrate masks the kinase site in the inactive kinase.
Binding of cGMP causes the kinase to flip open by the hinge thus exposing the kinase site to an authentic substrate.
The substrate can then bind to this site and is subsequently phosphorylated with the gamma phosphate group of ATP acting as the phosphate donor.
The phosphorylated substrate then leaves the kinase, ADP is replaced by ATP, and the kinase is ready to phosphorylate another substrate.
The kinase returns to the inactive form when cGMP leaves the nucleotide binding sites.
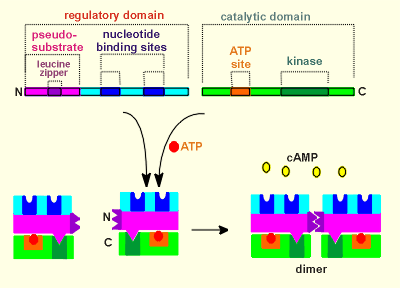 PKA has a similar structure to PKG but with an
important difference, namely it is the product of two genes.
PKA has a similar structure to PKG but with an
important difference, namely it is the product of two genes.
One gene codes for the regulatory subunit and another codes for the catalytic subunit.
There is no hinge domain.
As with PKG, the pseudo-substrate domain of PKA fits into the kinase domain.
PKA also possesses a leucine zipper, and thus two regulatory subunits are "fused" in the mature kinase.
The fact that the catalytic domain is a completely separate protein from the regulatory domain has important implications to the working of this kinase.
As the animation to the right illustrates, when PKA is activated by cAMP the catalytic units come completely separate from the regulatory domain.
These activated catalytic domains are then free to diffuse throughout the cell.
This gives PKA a much larger working range that PKG, which has to drag along its regulatory subunit.
Catalytic subunits of PKA have even been found in the nucleus of the cell where they can phosphorylate transcription factors.
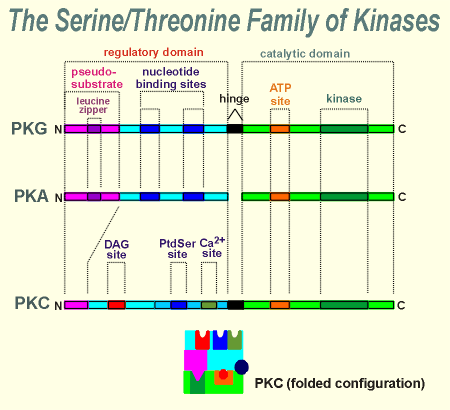 With PKC we return to a one-gene product, with a
hinge domain, similar to PKG.
With PKC we return to a one-gene product, with a
hinge domain, similar to PKG.
PKC lacks the leucine zipper in the regulatory subunit and thus it does not form a dimer.
For activation PKC requires diacyl glycerol (DAG), phosphatidyl serine (PtdSer) and Ca2+.
There is a site for each of these products in the regulatory domain of PKC.
As mentioned earlier, the name PKC comes from Ca2+ because it was first thought that Ca2+ was the critical factor activating this kinase.
It was subsequently shown that only low levels of Ca2+ are required to meet the Ca2+ requirements of this kinase.
Indeed, basal (resting) levels of Ca2+ are often sufficient, and thus it is the presence or absence of DAG which has proved to be the more important dynamic regulator of this kinase.
DAG is very hydrophobic and, following its synthesis, stays in the membrane.
Thus, not surprising, PKC usually functions near the membrane.
In fact, DAG is though to draw the kinase into a close association with the membrane, as illustrated in the movie clip to the left.
There is also a membrane bound protein called "Receptor for Activated C Kinase" (RACK), which is thought to be important in anchoring the activated kinase in the membrane.
Notice in the animation that while both phosphatidyl serine (PtdSer, a phospholipid) ) and Ca2+ (green ball) are necessary for kinase activity, it is DAG that is determining kinase activity.
The PtdSer and Ca2+ are normally present at sufficient levels to support enzyme activity.
This is not true for DAG, and thus it is the most important factor in determining PKC activity.
 To
understand the working of this family of kinases one must first have a basic
understanding of the ubiquitous Ca2+ binding protein calmodulin.
To
understand the working of this family of kinases one must first have a basic
understanding of the ubiquitous Ca2+ binding protein calmodulin.
Use the link to the right for a quick review of the structure of calmodulin.
There are many potential substrates for calmodulin, including the CaM kinases.
The best known kinase in this regard is Ca2+-calmodulin-dependent protein kinase II, often abbreviated CaMKII.
This kinase is extremely important for the functioning of the brain, where it accounts for 1-2 % of the total protein content!
Given to the left is the structure of CaMKII.
Its structure is similar to PKG, PKA and PKC but the regulatory domain is located in the C-terminal region and the catalytic domain in the N-terminal region.
Like the other kinases it possesses an ATP binding site near the kinase domain.
It also possesses a hinge domain.
With folding the so-called inhibitory domain or pseudo-substrate domain comes to rest in the vicinity of the kinase domain, thus ensuring that its activity is blocked.
CaMKII also has a phosphorylation site which, as you will see in a series of movies below, is very important to the functioning of this kinase.
Play the movie to see how the folding can bring the various functional domains of the kinase in the correct orientation.
To the right and below are a series of movies showing how Ca2+-calmodulin dependent protein kinase II works.
The first movie shows activation under conditions where there is only a very short duration increase in Ca2+.
The movie takes you through the activation of CaMKII step by step (press "play" button each time you want to advance a step).
Note that under the conditions of a short duration increase in intracellular Ca2+ the CaMKII activity is, and remains, directly dependent on Ca2+-calmodulin.
In other words, if there is no Ca2+-calmodulin present, the kinase is inactive.
When the Ca2+ returns to low basal levels the ions quickly dissociate from the calmodulin and the protein dissociates from the kinase.
The kinase then quickly returns to the closed, inactive, state.
To the left is the same movie, only rather than having to step through it, it plays without interuption.
Watch this move several times to get a feeling for what occurs with CaMKII under conditions of short duration increases in intracellular Ca2+.
Then go on to the move below, which shows what happens under more sustained increases of Ca2+.
The movie to the right shows what happens to CaMKII when the Ca2+ level remains high for a longer time.
In this case the enzyme is in the open state sufficiently long to be phosphorylated by another CaMKII.
The kinase cannot phosphorylate itself, thus this is not an autophosphorylation but a transphosphorylation.
In the movie note how the phosphorylated CaMKII tries to return to the inactive state but can't due to the phosphorylation.
This is the reason this kinase has been referred to as the kinase with a memory i.e. it remembers having been activated by Ca2+-calmodulin, and thus remains activated.
How the memory of CaM Kinase can be useful
The fact that CaMK has a "memory" can be useful because it can preform work long after an event has occured.
In considering this, lets look again at the Ca2+ microdomain and consider how the message that Ca2+ influx has occured gets out of the microdomain to alter functions deeper in the cell.
We have looked at this issue earlier, and seen that a message can get out of Ca2+ microdomains via Ca2+-calmodulin or Ca2+-induced-Ca2+ release (review Ca2+ domains?).
With CaMK we can add to this repertoire of mechanisms.
The movie to the left shows how.
In the case where Ca2+-calmodulin itself is carrying the message (first option in movie), a message with a very short half-life is generated.
Once the Ca2+-calmodulin is out of the microdomain the bound Ca2+ would be quickly lost, perhaps even before the Ca2+-calmodulin has found an appropriate effector molecule to activate.
If CaMK is also present in the microdomain, however, then Ca2+-calmodulin-activated CaMK (option 2 in movie) could also be carrying the message.
This might extent the half-life of the message somewhat, but as soon as the calmodulin looses its Ca2+ then the kinase will return to the inactive condition.
If the Ca2+ domain was present for a sustained period of time then transmolecular phosphorylations of CaMK within the domain would take place.
In this case the phosphorylated CaMK could become the carrier of the message out of the microdomain (option 3 in movie).
 From what you
have learned about CaMKII in the earlier movies it should be evident that this latter
option would generate a message with a long half-life i.e. this is now the kinase
with a memory.
From what you
have learned about CaMKII in the earlier movies it should be evident that this latter
option would generate a message with a long half-life i.e. this is now the kinase
with a memory.
The long half-life is believed to be important for the function of Ca2+-CaM Kinase II in many presynaptic nerve terminals.
Use the link to the right to see how this mechanism of long-term activation is important in nerve terminals of dopamine neurons.
In considering how phosphorylation of CaMKII can be a useful mechanism, consider what would happen if two CAMKII molecules were complexed or tied together.
The chances or probability of one CaMKII finding the other, for transmolecular phosphorylation, would be drastically increased in comparision to completely free enzymes.
If three kinase molecules were in the complex then the probability of transmolecular phosphorylation is increased even further.
In fact, CaMKII often exists in a complex consisting two rings, each ring containing six CaMKII molecules.
Thus the complex contains a total of 12 kinase molecules, i.e. it is a dodecameric holoenzyme.
The animation to the left gives an impression of the structure of such a complex.
The complex is held together via so-called "self-association" domains, which are found towards the C-terminal of the kinases, in a region which contains variable inserts.
Only isoforms of the enzyme which contain the association domains participate in forming the complexes.
If a cell makes such isoforms depends on how it alternatively splices the primary mRNA transcript during gene expression of the CaMKII gene.
Splicing in appropriate inserts will lead to the construction of a dodecameric CaMKII holoenzyme.
The self-associaton domains form a narrow stalk to the central mass of the complex.
This complex can become an integrating machine.
This is illustrated in 3 movies, given below, which show the working of one of the rings of the CaMKII complex under different conditions.
The movies use different symbols than the previous movies to represent the various compontents of the CaMKII system.
The symbols used are illustrated to the right.
The first movie shows the complex being activated under low, weak (or perhaps low frequency) Ca2+ signals, the second shows its activation under moderate Ca2+ signals and the third under high (or perhaps high frequency) Ca2+ signals.
In these movies an activated kinase within the ring can transphosphorylate its neighbour kinase in a clockwise direction.
Of course, in order to phosphorylate its neighbour two criteria must be meet.
First, the kinase itself must be active.
Secondly, its countrclockwise neighbour must be in the open condition so that it can be phosphorylated (i.e. it must also be activated by Ca2+ calmodulin).
Under low Ca2+ conditions the chances of two neighbouring kinases being activate by Ca2+-calmodulin (purple balls in movie) at the same time is very low.
Thus, under low Ca2+ activation no transphosphorylation takes place and the enzymes remain dependent on Ca2+-calmodulin for their activity.
As soon as Ca2+ levels drop (given as "no Ca2+" in the movies) the enzymes become inactive.
Under a moderate Ca2+ signal the probability of fullfilling the criteria is increased.
Now, during the time that Ca2+ is elevated, some of the enzymes of the holoenzyme become transphosphoylated.
This is indicated in the move by an arrow going from the catalytic site of one enzyme and depositing a "phospho-group" on its clockwise neighbour.
Once phosphorylated the enzyme remains active, even after Ca2+ has returned to basal values (again, given as "no Ca2+" in the movie)
Finally we come to the high Ca2+ signal.
Now we have a much higher probability of meeting the two criteria for transphosphorylation.
Notice that as more enzymes become phosphorylated (and thus permanently activated) the probalbility of a newly activated enzyme itself becoming phosphoylated increases.
This is simply because the probability that its neighbour is active has increased.
If the Ca2+ signal is strong enough then all enzymes of the holoenzyme become phosphorylated and thus remain active after Ca2+ returns to basal levels.
The CamKII holoenzyme is important in integrative processes.
For example, it can convert different frequencies of Ca2+ oscillations into different cellular responses.
In the above movies the low, moderate and high Ca2+ could in fact represent low, moderate and high frequencies of Ca2+ oscillations.
Likewise, the strong integrative activity of CaMKII holoenzymes are known to be important in the construction of memory in the brain.
It is evident that each of the four kinases (PKA, PKG, PKC and CaMKII) discussed above has unique signaling characteristics.
For example, PKA has catalytic units that come loose from the regulatory domain and are thus free to roam throughout the cell.
PKC requires multiple activators (PtdInos and Ca2+ in addition to DAG) and is activated only near the cell membrane.
Ca2+-CaM Kinase II has the distinction of having a memory, the kinase being active until a protein phosphatase has removed a phosphate group in the regulatory domain of the kinase.
This kinase can form an integrative machine when constructed into a large holoenzyme.
The presence of diverse second messenger driven kinases, each with unique signaling characteristic, adds considerably to the signaling potential of G protein coupled receptor mechanisms.
 Very often
the proteins being phosphorylated (or dephosphorylated) are rate-limiting enzymes in
metabolic pathways.
Very often
the proteins being phosphorylated (or dephosphorylated) are rate-limiting enzymes in
metabolic pathways.
In this regulation the phosphatases are equally important as the kinases, although the phosphatases are less well understood (use the link to the right to learn a bit more about the serine/threonine phosphatases).
By controlling the activity of the rate-limiting enzyme the signaling system can control the rate of the metabolic pathway.
In the example for the biosynthesis of dopamine (given as a link, above), tyrosine hydroxylase (TH) is the rate-limiting enzyme in the pathway leading to dopamine production.
By controlling the phosphorylation status of this enzyme the signaling system can regulate the pathway for dopamine synthesis.
Another principle is that the signaling system often amplifies through the enzymes.
Again, in the above example, each activated TH may catalyze the production of several hundred or even thousands of dopamine molecules before the TH is finally inactivated.
 The example
often cited for amplification is the regulation of the cellular program of the liver cell,
namely glycogenolysis (review cellular programs?)
The example
often cited for amplification is the regulation of the cellular program of the liver cell,
namely glycogenolysis (review cellular programs?)
The link to the left will explain how an amplification factor of about 10,000,000,000 is achieved when noradrenaline induces glycogen breakdown.
 In the first unit of this tutorial the
functioning of the ionotropic neuron was considered.
In the first unit of this tutorial the
functioning of the ionotropic neuron was considered.
The ionotropic neuron is the neuron working through the action of ion channels (review working of neuron through ion channels?)
Superimposed on each neuron is a second functional system, namely G protein-coupled receptors and their associated effector systems.
This system has been termed metabotropic because it often has far reaching effects on the metabolism of the neuron.
The types of protein being phosphorylated are often receptors, ion channels and other proteins important to the signaling function of the neuron.
One of the primary functions of the metabotropic mechanisms is regulating the synaptic strength between neurons (i.e. regulating presynaptically how much neurotransmitter is released with each action potential and, postsynaptically, regulating how sensitive the neuron is to the neurotransmitter).
Changing synaptic strength between neurons is thought to be the molecular and cellular basis of learning and memory.
There is good evidence that metabotropic mechanisms play a major role in learning and memory (this topic is discussed in detail in the lectures).
The metabotropic mechanisms generally work much slower than the ionotropic mechanisms.
The ionotropic EPSPs and action potentials occur within 1 or 2 ms.
Phosphorylation induced by metabotropic mechanisms take many seconds or minutes to occur.
The EPSPs are over within a few milliseconds whereas phosphorylations last many minutes or even hours (depending on the protein phosphatase status of the neuron).
The consequences of the phosphorylations can last many days or even years.
The latter can be the case when the targets of the second messenger activated kinases are transcription factors which induce gene expression (regulation of gene expression is considered in the last unit of the tutorial).
Thus, metabotropic mechanisms are as essential to nerve cell function as the more widely appreciated ionotropic mechanisms.