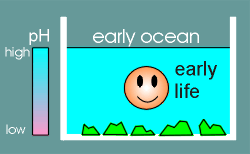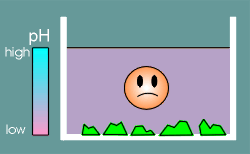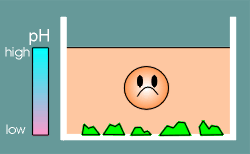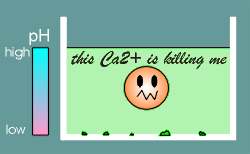
 Early in the evolution of life the worlds oceans were quite alkali.
Early in the evolution of life the worlds oceans were quite alkali.How does something so common as calcium become an important second messenger in the cell.
To understand this we have to look at the evolution of life itself.
 Early in the evolution of life the worlds oceans were quite alkali.
Early in the evolution of life the worlds oceans were quite alkali.
At this point in time most of the calcium was locked up in the form of precipitated salts (green blocks in figure to right).
Life produced CO2 and CO2 caused the gradual acidification of the ocean (this has been described as the first example of environmental pollution!).
With acidification the oceans gradually started to dissolve the calcium salts and consequently the concentration of Ca2+ increased.
Life that had been designed to operate under low Ca2+ suddenly found itself under high Ca2+.
Why is that a problem?
It is a problem because Ca2+ is a poison!
This may because Ca2+ is very similar to Mg2+.
Mg2+ in low amounts is important in binding to and giving shape to various regulatory proteins and DNA.
At high concentrations Ca2+ can substitute for Mg2+ but the shapes induced are not correct....consequently important functions fall away and the cell dies.
Also, at high concentrations Ca2+ forms a precipitable salt with phosphate, which has disastrous consequences to the cell.
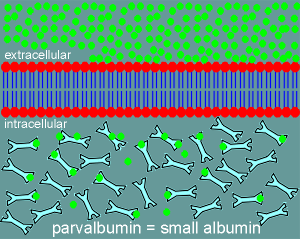 To overcome these problems the cell responded in two ways.
To overcome these problems the cell responded in two ways.
It developed Ca2+ binding proteins, such as parvalbumin, which it put in the cytosol to act as Ca2+ buffering proteins.
Parvalbumin (meaning small albumin, molecular weight ~10,000 daltons) is a low capacity (only 2 Ca2+ binding sites per molecule) high affinity Ca2+ binding protein.
It is present in extremely high concentration in the cytosol and consequently it can absorb high levels of Ca2+.
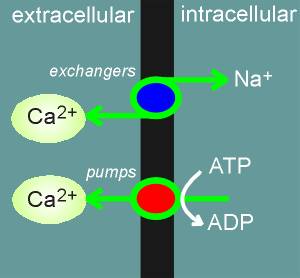 The cell also developed Ca2+ pumps (Ca2+-ATPases) and exchangers
to push Ca2+ out of the cell against a concentration gradient.
The cell also developed Ca2+ pumps (Ca2+-ATPases) and exchangers
to push Ca2+ out of the cell against a concentration gradient.
It also uses these pumps to push the Ca2+ into compartments within the cell such as into the endoplasmic reticulum (ER) and mitochondria.
In the endoplasmic reticulum (ER) it also placed Ca2+ binding proteins (calreticulin, calsequestrin) to "sop up" the Ca2+.
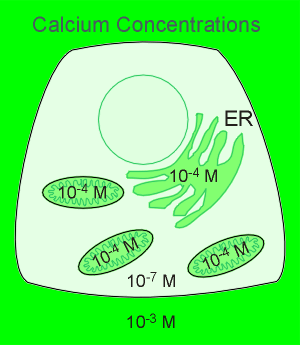 The result of all this is a high Ca2+ in the extracellular environment,
in the mitochondria and in the lumen of the ER.
The result of all this is a high Ca2+ in the extracellular environment,
in the mitochondria and in the lumen of the ER.
The cytosol has an extremely low Ca2+ concentration, some 10,000 times lower than what is found in the extracellular space.
Having developed mechanisms to hold the cytosolic Ca2+ at low levels, the way was clear to use Ca2+ as a message molecule...simple put in Ca2+ channels to give a controlled release of the ion!
Thus came voltage-operated Ca2+ channels on the membrane (a whole family with names like, N, L, P and Q etc.).
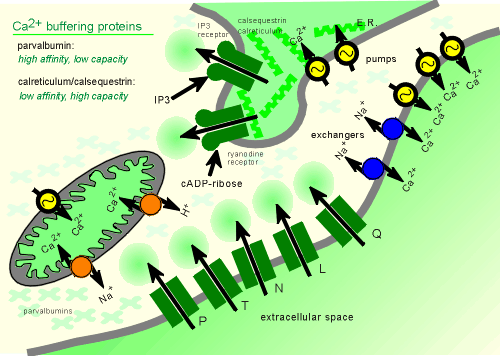
Also, Ca2+ channels were developed on the membrane of the ER..these are opened by intracellular second messengers IP3 and cADP-ribose.
IP3 is generated through the the enzyme phospholipase C (PLC), the activity of which is regulated by G protein coupled receptors (skip ahead to view generation of IP3 and diacylglycerol?)
Less is known about cADP-ribose.
The receptor for cADP-ribose is also acted upon by ryanodine, a product derived from plants (low concentrations of ryanodine activate and high concentrations inhibit the channel)
This Ca2+ channel has been given the name ryanodine receptor.
Finally, as a third class of Ca2+ channels, we have a ligand-operated Ca2+ channels on the membrane, namely the NMDA receptor.
 In
the illustration to the right is a side view of a Ca2+ channel, ,such
as an NMDA receptor or voltage-gated Ca2+ channel, with the front
subunit removed to view the inside channel.
In
the illustration to the right is a side view of a Ca2+ channel, ,such
as an NMDA receptor or voltage-gated Ca2+ channel, with the front
subunit removed to view the inside channel.
As soon as Ca2+ enters a cell (green in the animation) it is either mopped up by the Ca2+ buffering proteins or pumped out of the cell (or into an intracellular compartment).
Thus Ca2+ can not diffuse very far from its site entrance.
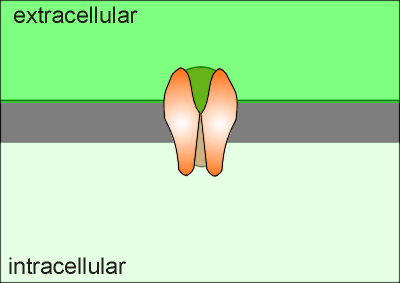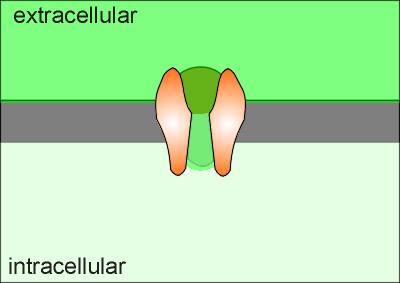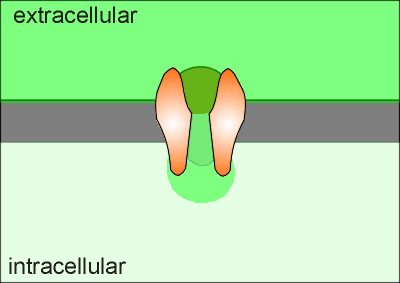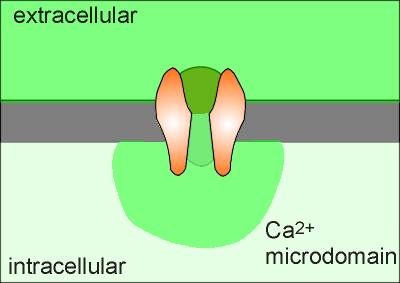
Consequently, when the channel opens it forms what is called a "Ca2+ microdomain" around the opening of the Ca2+ channel.
If Ca2+ is to act as a signal molecule then it must signal within this domain or find a way of carrying the signal outside of the domain.
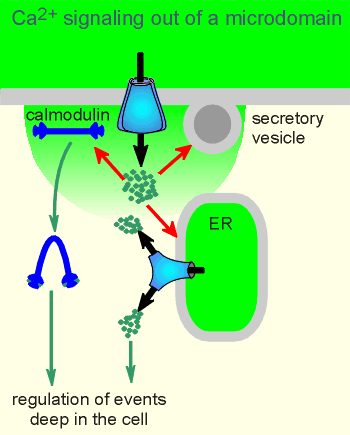 In its function in regulating exocytosis the secretory granules
are often found within the domain.
In its function in regulating exocytosis the secretory granules
are often found within the domain.
For carrying the signal outside of the domain two strategies have evolved.
One is to activate the protein calmodulin which can then diffuse out of the microdomain to regulate the activity of yet other proteins (there is more on calmodulin in the main body of this tutorial module, and calmoduin and Ca2+ microdomains are discussed further in a later module (skip ahead to see a movie of calmodulin signaling out of a microdomain?).
The other strategy is dependent on a very special property of IP3 and ryanodine receptors, namely that they also function as receptors for Ca2+.
Ca2+ induces opening of IP3 and ryanodine receptors in a process know as Calcium-Induced Calcium Release (CICR).
Through the CICR mechanism the Ca2+ signal can be propagated to deep within the cell.
Ca2+ increases through the action of ion channels on the membrane is referred to as "Ca2+ influx" whereas Ca2+ coming from IP3 or ryanodine receptors on the ER is referred to as "Ca2+ mobilization".
To the right is a movie showing Ca2+ mobilization via CICR.
The movie shows how CICR can create a wave of Ca2+ by acting on IP3 receptors.
To create a wave the Ca2+ released by one channel activates a neighbouring channel, which in turn activates and third channel etc.
In this way a wave can be sent all the way into the nucleus, where it is believed that there are also Ca2+ release channels to give rise to the nuclear Ca2+ signal.
In the nucleus there are Ca2+ binding proteins to convert the Ca2+ signal into useful work such as gene expression.
Important to the understanding of CICR is the fact that Ca2+ has a biphasic effect on the IP3 (and ryanodine) channel.
Low Ca2+ causes opening of the channel but as the level of Ca2+ increases the Ca2+ has an inhibitory effect on the channel.
Thus, as Ca2+ levels increase the channel opens but finally the Ca2+ concentration reaches a level where it is inhibitory and the channel closes.
This is why, in the movie, the channels are closing after their activation.
In this movie a voltage-operated Ca2+ channel (VOCC) on the membrane was responsible for initiating the CICR and the wave.
Later, you will see this same move, only IP3, generated by the enzyyme phospholipase C (PLC) will be responsible for the mobilization of Ca2+ and the initation on the wave (skip ahead to view movie?)