 Signaling
domains on the membrane
Signaling
domains on the membrane Signaling
domains on the membrane
Signaling
domains on the membraneIt was once thought that membrane bound proteins (such as receptors, G proteins, effectors etc.) were free to float over the entire membrane (like a boat on the sea).
This was called the fluid mosaic model of the membrane (mosaic because the membrane would be a random mosaic of different proteins).
It is now becoming clear that there is considerable structure to the membrane whereby membrane bound proteins are restricted in their movements.
Proteins can form signaling domains or supramolecular signaling complexes on the membrane, the properties of which depend on which proteins are found within the domain.
While it is not completely understood how the constraints on movement are achieved, a few mechanisms are known and have been depicted in the figure below.
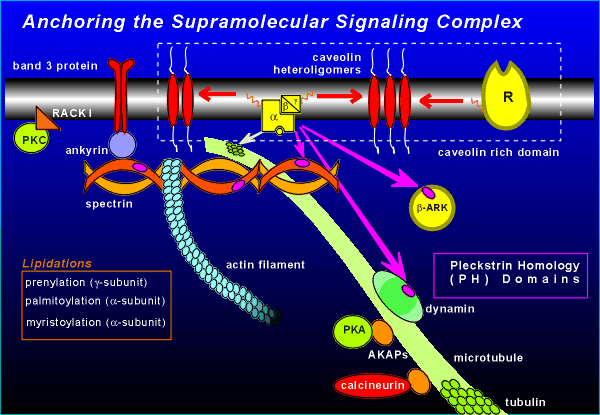 Cell membranes have protein rich patches composed
of the oligomers of the protein caveolin.
Cell membranes have protein rich patches composed
of the oligomers of the protein caveolin.
These patches are referred to as "caveolin rich domains".
In the isolation of caveolin it has been found that proteins of G protein signaling (G proteins, receptors) are often co-isolated with the caveolin.
Apparently these proteins have an affinity for caveolin.
This attraction is thought to be via lipid groups which are attached to the receptors and G proteins (indicated by the orange lines in the figure).
These lipidations are added as a post-translational modification (the lipid moiety is added to the side-chain of certain amino acids within the proteins).
There are different types of lipidations involved (see figure), the common feature among them being the creation of an affinity for caveolin.
Components of the signaling complex can also be attached to the cytoskeleton of the cell.
For example, the alpha subunit of G proteins shows an affinity for the protein tubulin, the important building block protein of microtubules.
The beta subunit shows affinity for a pleckstrein homology domain (PH domain) found in the cytoskeletal protein spectrin.
PH domains are known to be involved in protein-protein interactions (similar to SH2 and SH3 domains; review protein-protein interactions via SH2 and SH3 domains?)
The name PH domain designates a sequence of about 100 amino acids first found in pleckstrein, a protein found in erythocytes.
PH domains are also found in dynamin, one of the proteins involved in endocytosis, which could be involved in receptor desensitization or down-regulation (i.e. receptor internalization) (review receptor down-regulation, desensitization?)
Of clear functional significance to receptor signaling is the PH domain of beta-ARK (beta-adrenergic receptor kinase, also known as G protein receptor kinase, GRK).
It is believed that the beta/gamma subunit is targeted to beta-ARK via its PH domain (review activation and function of beta-ARK/GRK?).
The protein spectrin can also be involved in holding supramolecular protein complexes in place on the membrane via the protein ankyrin.
Downstream components of the signaling system (e.g. the kinases) can also become anchored.
We have already discussed RACK I which anchors activated PKC to the appropriate place in the membrane (review activation of PKC?)
Protein kinase A (PKA) also has anchor proteins referred to as A Kinase Anchoring Proteins (AKAPs).
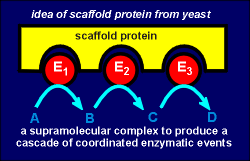 AKAP is an example of a so-called scaffold
protein, a term first coined to describe proteins in yeast which hold arrays of enzymes in
an appropriate configurations for efficient catalysis (see fig. to right).
AKAP is an example of a so-called scaffold
protein, a term first coined to describe proteins in yeast which hold arrays of enzymes in
an appropriate configurations for efficient catalysis (see fig. to right).
Another example of a scaffold protein was considered earlier, namely the post-synaptic density protein PSD-95.
This was the protein which held the NMDA receptor in the synapse (review anchoring receptors in the synapse with PSD-95?).
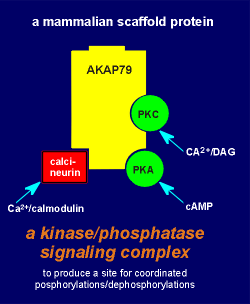 Similar to PSD-95, AKAPs
have multiple sites for protein-protein interactions.
Similar to PSD-95, AKAPs
have multiple sites for protein-protein interactions.
One of the AKAPs, AKAP79 (Mr 79,000 daltons) anchors not only PKA but also PKC and calcineurin, a Ca2+/calmodulin-regulated protein phosphatase (fig. to left).
The inactive PKA is bound to AKAP via its regulatory subunit (review structure of PKA?).
The AKAP in turn can be attached to other cell components such as the microtubules.
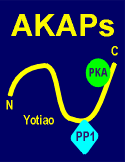 Upon activation the
kinase comes loose from the AKAP, and the catalytic component is free to phosphorylate
target protein in the vicinity of the anchoring point.
Upon activation the
kinase comes loose from the AKAP, and the catalytic component is free to phosphorylate
target protein in the vicinity of the anchoring point.
In fact AKAP is proving to be a large family of diverse proteins.
The link to the right shows how AKAPs are used to station PKA near various PKA targets, including ion channels.
One of the consequences of signaling domains is that the cell, through the construction of domains, can decide what its response will be to activation by particular receptor mechanisms.
For example, consider the two theoretical signaling domains shown in the figure below.
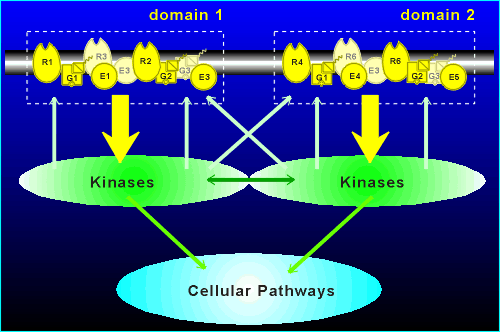 Suppose E1 (effector 1) is
adenylyl cyclase I (AC I) while E4 is a G(i) activated K+ channel
Suppose E1 (effector 1) is
adenylyl cyclase I (AC I) while E4 is a G(i) activated K+ channel
R1 activates G1 (= G(i)) to cause an decrease in cyclic AMP production while R2 activates G2 (also = G(i)) to activate the K+ channel.
By constructing domains the cell has decided that the response to R1 activation is a decrease in cyclic AMP and the response to R4 activation is hyperpolarization.
If there were no domains, and all proteins were free to interact with all other proteins, then no clean unambiguous response would be possible with R1 and R2 activation.
R1 would both lower cyclic AMP and cause hyperpolarization through K+ channel activation, as would R4!
The constraints of domain structure has added to the versatility of the G protein coupled receptor mechanisms to generate diverse signals.
The domains can communicate via the downstream kinases (i.e. kinases activated by domain 1 could diffuse into and phosphorlyate signaling components of domain 2, and vice versa) and they could feed into common cellular pathways.
The types of domains a cell constructs is laid down during cell differentiation.
These domains would be developed during evolution so that the cell can make the appropriate response to activation by neuropeptides, neurotransmitters and peptide hormones.
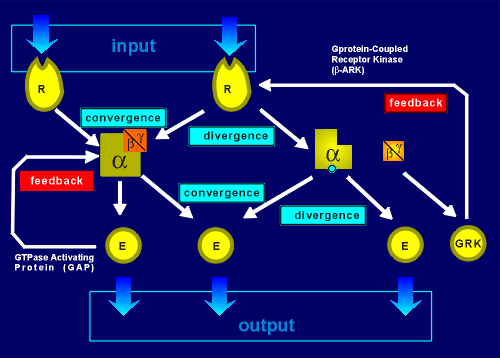 The system is even
more versatile than indicated above because, within each domain, a tremendous amount of
integration is possible.
The system is even
more versatile than indicated above because, within each domain, a tremendous amount of
integration is possible.
For example, two receptors may converge on the same G protein and thus the G protein would integrate the response.
A single receptor may diverge, activating more that one type of G protein within a domain.
Likewise G proteins can converge on an effector (e.g. perhaps G(i) and G(s) on adenylyl cyclase type I).
Or, a G protein can cause divergence (e.g. consider if both adenylyl cyclase type I and the G(i)-activated K+ channel are both in the domain).
Convergence and divergence arecharacteristics of neural networks and yet, what we are discussing here is all occurring in a tiny domain on the membrane of a neuron.
Added to this, feedback systems are built into the signaling complexes.
One feedback is via G protein-coupled receptor kinases, which has already been discussed (review GRKs?).
The other is GAP proteins which are special down-stream effectors that stimulate the GTPase activity of G proteins, thus pushing them into their inactive GDP-bound form.
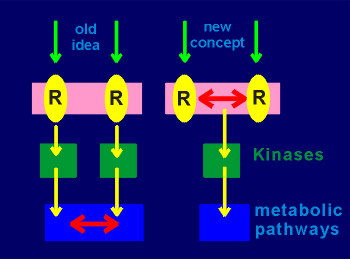 To conclude......
To conclude......The old idea for the working of metabotropic mechanisms was that the receptors would pass along relatively unprocessed input to the down-stream kinases.
The kinases in turn would act on metabolic pathways where signal integration would finally occur (integration is indicated by the red double arrows in the figure to the right).
In the concept of supramolecular signaling complexes and signaling domains the idea is that a considerable amount of the integration of signal input has already occurred at the level of the membrane.
This would occur through the convergence and divergence processes that occur in the signaling domains on the membrane.
Signaling domains almost certainly exist, but the evidence for them is mostly indirect or circumstantial.
One piece of indirect evidence is the remarkable observation that G protein-coupled receptors are quite promiscuous when one considers which G protein they will interact with!
This was discovered when molecular methods allowed the receptors to be expressed in e.g. Xenopus oocytes.
Express the beta-adrenergic receptor together with G(s) and one obtained, as expected, a receptor complex which, when activated with noradrenaline, would activate adenylyl cyclase.
However, coexpress the receptor with G(i) and one obtains, to everyone's surprise, a signaling complex which, when activated with noradrenaline would inhibit adenylyl cyclase!
The implication is that normally the beta-adrenergic receptor is placed in domains with G(s) and thus its apparent ability to activate G(i) is never seen.
In fact, while receptors generally have a preference for one G protein over another, they tend to be promiscuous if the opportunity arrises (such as in the case where they are expressed in Xenopus oocytes with inappropriate G proteins).
 Likewise, it
has been found that disruption of membrane structure through treatment of cells with mild
detergents can cause seemingly inappropriate receptor G protein responses.
Likewise, it
has been found that disruption of membrane structure through treatment of cells with mild
detergents can cause seemingly inappropriate receptor G protein responses.
For example, acetylcholine muscarinic receptors in the brain couple poorly with G(0) but if the membrane is transiently treated with detergent then they interact strongly with G(0).
The implication is that membrane disruption caused mixing of signaling domains such that, in the above example, the muscarinic receptor came into the same domain as G(0).
Finally there are a number of experiments where the most logical explanation for the results is the operation of signaling domains on the membrane.
The link above gives an overview of one such example.
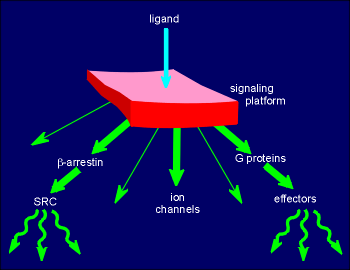 G proteins coupled
receptors got their name for their ability to activate G proteins.
G proteins coupled
receptors got their name for their ability to activate G proteins.
Recent research has shown that these receptors can signal through many other mechanisms besides via the G proteins.
In fact GPCRs can be thought of as signaling platforms which, depending on the receptor, the cell type and the repitoire of partner proteins in the immediate vacinity, can signal through many different mechanisms.
The illustration to the right shows some of these signaling possibilities, where the GPCR is represented as the red platform.
The platform can signal through protein-protein interaction with G proteins, the subject of this tutorial.
As well, ligand activation of the receptor can induce it to bind to and modify the function of ion channels on the membrane.
Another alternative mechanism of signaling is via beta-arrestin, the molecule that binds and inactivates GPCR interaction with G proteins (review the action of G protein coupled receptor kinase, GRK?)
It has now been shown that beta-arrestin can bind to and activate the cytosolic tyrosine kinase Src (review Src?) in a tyrosine kinase receptor independent way.
These are just a few of the many ways these highly versatile receptors can signal.
Details on these alternative mechanisms of signaling is beyond the scope of this tutorial.