
These substrates then go on to alter cell function (step 5 right).
Thus, the function of each tyrosine kinase receptor depends on the function of the substrates they phosphorylate (and thus activate).
An analysis of all the TKR substrates, or even all the substrates for a single receptor, is beyond the scope of this tutorial.
Therefore, to give an impression of the function of tyrosine receptor kinases, a selection has been made.
This selection has been made from among the various substrates for the Platelet Derived Growth Factor (PDGF) receptor.
This receptor has already been introduced in the module TKR Structure as a prototype for TKR receptors.
 Shown to the left is, again, the intracellular domain of the PDGF receptor.
Shown to the left is, again, the intracellular domain of the PDGF receptor.
This shows the location of the important tyrosine groups (blue balls) in the C-terminal of the receptor that form binding sites (when phosphorylated on the hydroxyl side group) for the substrates.
The PDGF substrates that will be considered in further detail are cSrc, GAP and PLC-gamma.
These contain the so-called SH2 regions to bind to phosphorylated tyrosines.
The binding position of these substrates to the PDGF receptor are indicated in the figure to the left.
Keep in mind that these substrates bind to activated tyrosine kinase receptors where they themselves then become phosphorylated and thus activated.
Given below is an outline of what these three important effector molecules do in the cell.
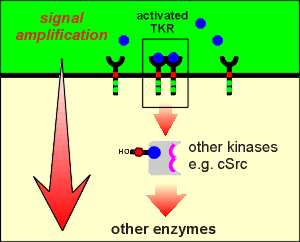 Cellular Src or, cSrc, is a non-receptor protein kinase.
Cellular Src or, cSrc, is a non-receptor protein kinase.
It was originally discovered as the protein product of a viral oncogene (tumor generating gene) called Src.
With the discovery of a cellular counterpart the viral product was designated vSrc and the cellular counterpart cSrc (there will be more information about oncogenes in a later module of this tutorial).
Src plays the role of a protein switch in the regulation of cell growth and cell division (thus it is not surprising that the viral version of the protein causes cancer).
There are now nine members of the Src family of tyrosine kinases.
Every eucaryotic cell studied to date has at least one of the family members as part of its intracellular signaling structure.
The PDGF receptor phosphorylates cSrc and cSrc then phosphorylates other intracellular proteins.
Enymyes activating other enzymes illustrates an important principle of signal transduction, namely amplification.
A very small amount of ligand can activate a few receptors....these few receptors, being enzymes, can activate a large amount of substrate...thus amplification.
If the substrate itself is an enzyme then we get yet another layer of amplification.
Intracellular kinases (both Ser/Thr kinases and non-receptor tryrosine kinases) are often found to be part of intracelluar amplifying cascades or chains (one kinase activating the next, which activates the next etc.).
In the case of Src this ultimately leads to cell growth and division.
Thus the ultimate target of Src is the genome.
The PDGF receptor is but one element in the regulation of cSrc.
The complete regulation of the enzymatic activity of cSrc has proved to be extremely complex (and beyond the scope of this tutorial....check out the web sites if you want more information on Src.)
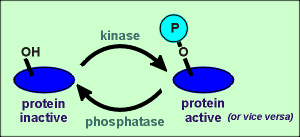
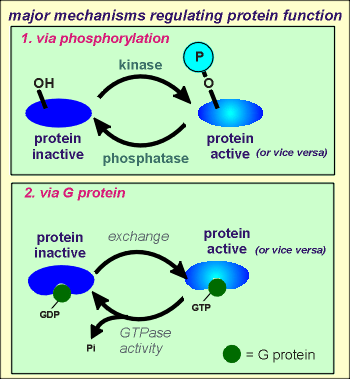 There are two major mechanisms regulating the activity of proteins in
eucaryotic cells.
There are two major mechanisms regulating the activity of proteins in
eucaryotic cells.
One of these mechanisms is the subject of this tutorial, namely phosphorylations of hydroxyl (OH) groups of the protein through protein kinases (e.g. tyrosine kinases).
The other mechanism is through interaction with G proteins, so-called because the proteins are GTPase (they hydrolyze GTP to produce GDP).
G protein will be a major topic in the G protein-coupled receptor modules of the tutorials.
When G proteins are in the GTP bound form they can bind with and activate (or in some cases inacitvate) other proteins (e.g. enzymes, ion channels etc).
In the GDP bound form the G protein is unable to affect the activity other proteins.
Thus, an exchange of a GDP for a GTP can activate the target protein and hydroylsis of the GTP to give GDP and inorganic phosphate (Pi) can inactivate the target protein (or vice versa).
Needless to say, the regulation of G proteins becomes a major target in mechanisms regulating cellular functions (e.g. G protein-coupled receptors regulate the activity of a class of G proteins, as you will see in later modules of the tutorials).
GAP is a family of proteins which, as the name suggests, stimulates the GTPase activity of G proteins.
In doing so the GAPs bring G proteins into the inactive GDP bound state, thus GAPs inhibit G protein signaling.
Through the activation of GAPs, tyrosine kinase receptors can be involved in the regulation of G protein signaling in the cell.
 Examples where G protein signaling is used in the regulation of
cellular events are:
Examples where G protein signaling is used in the regulation of
cellular events are:
(1) G protein-coupled receptors,
(2) G proteins associated with the biosynthetic machinery of the cell, and
(3) G proteins associated with the exocytotic machinery of the cell.
Thus GAPs play a central role in the coordination of cellular events.
Tyrosine kinase receptors can regulate the activity of GAPs and thus the receptors can participate in the regulation of many intracellular events.
 Phospolipase C (PLC) is a family of enzymes which breakdown the membrane
phospholipid phosphatidyl inositol 4,5P2 (PIP2).
Phospolipase C (PLC) is a family of enzymes which breakdown the membrane
phospholipid phosphatidyl inositol 4,5P2 (PIP2).
From this breakdown two intracellular second messengers are produced.
The lipid part of the phospholipid gives rise to diacylglycerol (DAG), which, being a lipid, remains in the hydrophobic environment of the membrane.
The sugar part of the phospholipid gives rise the phosphorylated sugar inositol 1,4,5 triphosphate (IP3).
This water soluble sugar comes into the cytosol.
The two second messengers activate kinases within the cell.
DAG directly activates Protein Kinase C (PKC), a Ser/Thr Kinase, which can then phosphorylate other enzymes (E = enzyme in the illustration).
In a later module of the tutorial, where the action of Ser/Thr protein kinases are examined in greater detail, there is a movie of DAG activation of PKC.
Use the link to the right, if you want to take a look at this movie now (skip ahead to the PKC movie?)
_______________
IP3 works indirectly in activating kinases.
First, IP3 acts on its receptor, a calcium channel on the membrane of the endoplasmatic reticulum, to cause an increase in intracellular Ca2+.
The action of IP3 is considered in greater detail in later module of the tutorials and again, a movie has been prepared.
Use the link to the right if you would like to view this movie now (skip ahead to the IP3 receptor movie?)
________________
The Ca2+ ions released from the IP3 receptor can activate Ca2+-induced Ca2+ release (CICR), as discussed in an earlier module of the tutorial (review CICR?) and shown in the above movie.
Also, the Ca2+ can bind to the Ca2+ binding protein calmodulin, the first step towards activating a kinase (the protein calmodulin will be examined in more detail later in the tutorials).
The Ca2+-calmodulin complex binds to and activates a Ser/Thr Kinase known as Calcium calmodulin dependent protein kinase.
Here too a movie has been prepared for a later module of the tutorials showing the activation of such a kinase by Ca2+-calmodulin.
Use the link to the right if you would like to view this movie now (skip ahead to the kinase movie?).
 The lipase activated by tyrosine kinase receptors is called PLC-gamma.
The lipase activated by tyrosine kinase receptors is called PLC-gamma.
This lipase is a member of a larger family which includes PLC-beta.
PLC-beta is activated by G protein-coupled receptors (thus the above movies concerning the actions of DAG and IP3 are included in the G-protein coupled receptor module of the tutorials, as is further discussion of calmodulin).
PLC-beta is a membrane bound form of the enzyme (see illustration to right).
PLC-gamma is cytosolic and thus can interact with activated tyrosine kinases to be phosphorylated and activated.
_________________
To summarize, tyrosine kinase receptors activate multiple intracellular effectors; these effectors initiate complex cascades of intracellular signaling events leading to dramatic changes in intracellular events.
This phenomenon, while observed for many different receptor types, is a particularly strong characteristic of tyrosine kinase-type receptors.
The first indication of the internalization process is the accumulation of receptors in coated pits, so-called because they are coated on the inside of the membrane with the matrix protein clathrin.
These pits invaginate to form coated vesicles which give rise to the endosomes when the clathrin is removed.
What is the purpose of this endocytosis of the receptor-ligand complexes?
Several ideas have been put forward.
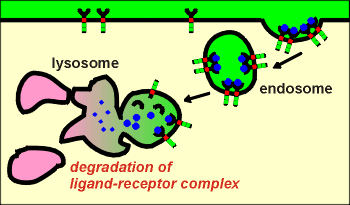 First idea: down regulation
First idea: down regulation
In this scenario the endocytosed ligand-receptor complexes are sent to the lysosomes where they are both degraded.
This has been shown to occur, particularly in cells that have been exposed to extremely high concentrations of ligand.
This can be seen as a mechanism for the cell to protect itself against over-stimulation by the ligand.
The cell is said to be "down regulated" with respect to a response to the ligand.
The cell can only regain its sensitivity to ligand through synthesis of new receptors, a process that takes many hours or days.
.
.
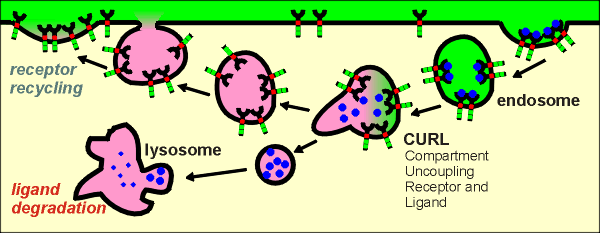 Second idea: desensitization
Second idea: desensitization In this scenario the endocytosed ligand and receptors of the ligand-receptor complexes are uncoupled.
This occurs through acidification of the endosome by the action of proton pumps on the endosome membrane.
At low pH the ligand can no longer bind to the receptors and thus becomes uncoupled from the receptor.
The ligands are sent to the lysosomes for degradation and the receptors are recycled to the membrane.
The cell undergoes a transitory "desensitizaton" to the ligand but can recover sensitivity within minutes because the receptors are returned to the membrane.
This mechanism has been shown to occur at physiological concentrations of ligand.
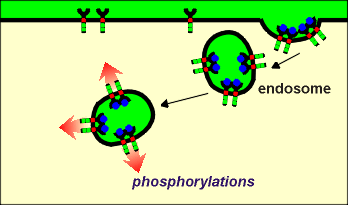 Third idea: a
new form of cell signaling
Third idea: a
new form of cell signaling During endocytosis extracellular fluid is taken up in the endosome.
This fluid is an appropriate medium for ligand binding to the receptors...thus one would expect that the ligand-receptor complexes would remain intact until some active process (such as proton pumping mentioned above) causes their dissociation.
Thus, the endosomes would have active catalytic sites protruding into the cytosol.
These catalytic sites could phosphorylate intracellular substrate proteins.
There is recent evidence that indeed endocytosed receptors may be active.
In neurons it has been suggested that endocytosed tryosine kinase receptors at nerve terminals may be transported to the cell body where they phosphorylate target proteins.
 Protein phosphatases can be divided into two main groups, Ser/Thr
phosphatases and tyrosine phosphatases.
Protein phosphatases can be divided into two main groups, Ser/Thr
phosphatases and tyrosine phosphatases.
While the phosphatases are equally important as the kinases in the regulation of intracellular events, far less is known about the regulation of the activity of the phosphatases.
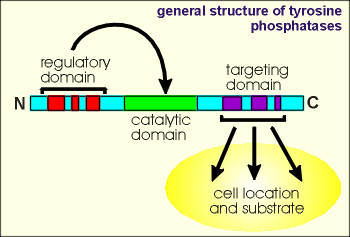 The tyrosine phosphatases are a family of proteins which
have a highly conserved catalytic domain (~250 amino acid residues) flanked by N- and
C-terminal segments which are involved in regulating catalytic activity or targeting the
the enzyme to particular cell locations and substrates.
The tyrosine phosphatases are a family of proteins which
have a highly conserved catalytic domain (~250 amino acid residues) flanked by N- and
C-terminal segments which are involved in regulating catalytic activity or targeting the
the enzyme to particular cell locations and substrates.
An important mechanism for controlling the activities of phosphatases is by phosphorylation.
This leads to intricate networks of positive and negative feedback regulatory cycles between kinases which activate phosphatases which in turn inactivate kinases.
Interestingly, a putative tyrosine phosphatase receptor has recently been described.
It has been proposed that it would transduce extracellular signals by dephosphorylating phosphotyrosine residues of intracellular substrate proteins.