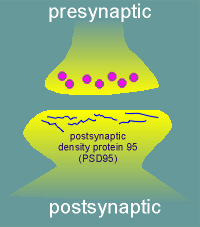
 A good candidate for an anchor was a protein that is found in
high density at the postsynaptic side of many synapses.
A good candidate for an anchor was a protein that is found in
high density at the postsynaptic side of many synapses.Receptors are highly concentrated in the synapse which has led some to propose that they are somehow anchored there.
 A good candidate for an anchor was a protein that is found in
high density at the postsynaptic side of many synapses.
A good candidate for an anchor was a protein that is found in
high density at the postsynaptic side of many synapses.
This protein has been given the name postsynaptic density protein 95 (PSD95; the 95 refers to its molecular weight of 95 kD).
Indeed, the glutamate NMDA receptor was found to bind to PSD95.
Sequencing of PSD95 through the recombinant approach revealed that this protein possesses two different types of domains known to be involved in protein-protein interactions.
One of these is called the PDZ domain and the other is called the SH3 domain.
PDZ and SH3 domains are domains within the proteins involved in protein-protein interactions
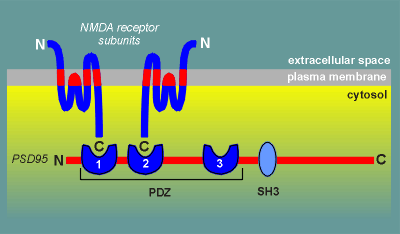 PSD95 contains 3 PDZ domains and 1 SH3 domain.
PSD95 contains 3 PDZ domains and 1 SH3 domain.
It has now been established that the NMDA receptor binds to the PDZ domains (the function of the SH3 domain has yet to be established).
The binding is through the C-terminal of the NMDA receptor subunits (remember, unlike most ion channel receptors, the C-terminal of glutamate receptor channels is intracellular; review structure of glutamate receptor?)
The first two PDZ domains of PSD95 have high affinity for NMDA receptor subunits.
The third PDZ domain has only low affinity for NMDA receptor subunits and thus is not thought to be involved in anchoring the NMDA receptor to the synapse.
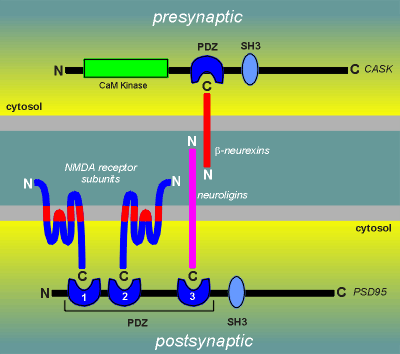 This third PDZ domain could very well be involved in helping
to hold the synapse together.
This third PDZ domain could very well be involved in helping
to hold the synapse together.
It has been found that the C-terminal of a family of proteins known as neuroligins, which are postsynaptic transmembrane proteins, can make high affinity binding to the 3rd PDZ domain.
The N-terminal of neuroligins are known to bind to the N-terminal of a presynaptic transmembrane protein family called the beta-neurexins.
The beta-neurexins in turn bind via a PDZ domain to a presynaptic protein called CASK (which also contains a kinase catalytic domain for the phosphorylation of calmodulin).
These protein-protein interactions can be thought of as a "glue" holding the pre- and postsynaptic membranes together.
There is even the possibility that the pre- and the postsynaptic compartments may somehow be able to communicate via these protein bridges.
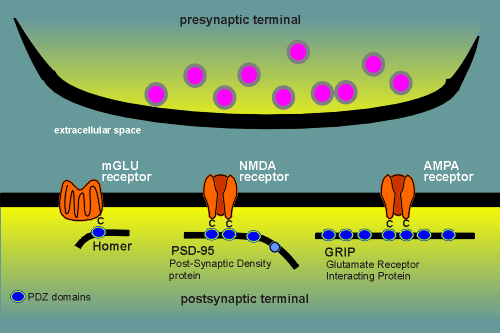 PDZ domains have proved to be involved in binding to a number
of glutamate receptors.
PDZ domains have proved to be involved in binding to a number
of glutamate receptors.
The glutamate AMPA receptor binds to a protein named Glutamate Receptor Interacting Protein or GRIP.
GRIP has 7 PDZ domains of which domains 4 and 5 are thought to be involved in binding the AMPA receptor.
The AMPA receptor binding is again via the C-terminal.
The metabolic glutamate receptor (mGLU) binds via the C-termina to a PDZ domain in a protein given the name Homer (presumably because it shows the receptor where its home is).
While among the receptors, the anchoring of glutamate receptors is the best understood, it would appear that other receptors are also anchored in the synapse through mechanisms similar to those displayed by the glutamate receptors.
For example, GABAa receptors and glycine receptors are believed to anchor to a cytoplasmic protein called Gephyrin.
Additional reading