 An interesting example of
an AKAP is the protein yotioa.
An interesting example of
an AKAP is the protein yotioa.AKAPs are a functionally related family of proteins which possess a conserved PKA binding domain.
The extreme N terminus of the regulatory domain of PKA bind to the AKAPs.
Each AKAP contains a unique targeting domain that directs the kinase/AKAP complex to a defined intracellular location.
 An interesting example of
an AKAP is the protein yotioa.
An interesting example of
an AKAP is the protein yotioa.
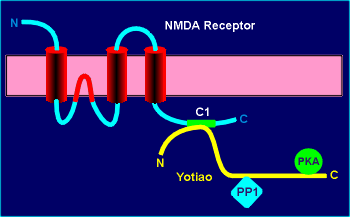
Yotioa binds not only PKA but also protein phosphatase type 1 (PP1).
Thus both a kinase and a phosphatase which act on the same substrate are colocalized in the same signaling complex.
Yotiao binds to the NMDA receptor in a segment near the C terminal that is coded for by the so-called C1 exon in the gene.
Enzymatic analysis suggests that yotiao tethers PP1 in the active state favoring dephosphorylation under resting conditions.
This attenuates receptor activity.
Elevated cyclic AMP releases the anchored kinase to phosphorylate and upregulate channel activity.
The NMDA receptor is tethered by its extreme C-terminal to PSD-95, a scaffold protein which likely targets other proteins to the NMDA receptor (review PSD-95?).
Thus through Yotiao and PSD-95 a very large supramolecular signaling complex is constructed around the NMDA receptor.
Many other ion channels have been either implicated or demonstrated to bind AKAPs.
These include the glutamate AMPA receptor, the L- and N-type voltage-operated Ca2+ channels, the Ca2+-activated K+ channel, and voltage-operated Na+ channels.
AKAP targeting of PKA and other enzymes to their target proteins seems to be a general phenomenon.