G protein-coupled receptor kinase (GRK)
A notable feature of G protein pathways is that the cell
surface receptors are subject to precise regulation of their sensitivity.
Prolonged or repeated exposure of a receptor to its ligand
(agonist) leads to diminished responsiveness of the receptor to further stimulation.
This has been termed receptor "desensitization".
One important mechanism for receptor desensitization
involves phosphorylation of the receptor by a kinase call G protein-coupled receptor
kinase or GRK.
Given below is the sequence of events which leads to
receptor desensitization by GRK.
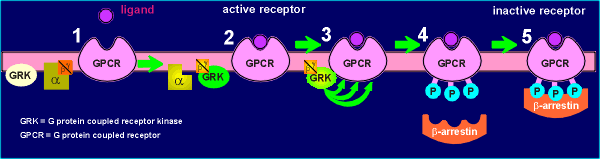 The situation before activation is given in (1). With
receptor activation (2) the G protein is activated and the beta/gamma subunit activates
GRK. The kinase then phosphorylates the receptor at 3 sites leading to the phosphorylated
receptor (4). The phosphorylated receptor then binds the protein beta-arrestin (5). The
beta-arrestin prevents prevents the receptor from activating more G proteins, thus giving
rise to an inactive or desensitized receptor.
The situation before activation is given in (1). With
receptor activation (2) the G protein is activated and the beta/gamma subunit activates
GRK. The kinase then phosphorylates the receptor at 3 sites leading to the phosphorylated
receptor (4). The phosphorylated receptor then binds the protein beta-arrestin (5). The
beta-arrestin prevents prevents the receptor from activating more G proteins, thus giving
rise to an inactive or desensitized receptor.
This mechanism was first discovered for the beta-adrenergic
receptor and thus the kinase responsible for receptor phosphorylation was called the
beta-adrenergic receptor kinase, or beta-ARK.
It was only later discovered that this same kinase in fact
phosphorylated many other G protein-coupled receptors.
Thus a better designation for the kinase would be G
protein-coupled receptor kinase or GRK (despite this many textbooks and articles still
refer to the kinase as beta-ARK).
This desensitization mechanism is very general for G
protein-coupled receptors and it is now know that GRK is a family of kinases with at least
six members (named GRK1 through GRK6).
 The situation before activation is given in (1). With
receptor activation (2) the G protein is activated and the beta/gamma subunit activates
GRK. The kinase then phosphorylates the receptor at 3 sites leading to the phosphorylated
receptor (4). The phosphorylated receptor then binds the protein beta-arrestin (5). The
beta-arrestin prevents prevents the receptor from activating more G proteins, thus giving
rise to an inactive or desensitized receptor.
The situation before activation is given in (1). With
receptor activation (2) the G protein is activated and the beta/gamma subunit activates
GRK. The kinase then phosphorylates the receptor at 3 sites leading to the phosphorylated
receptor (4). The phosphorylated receptor then binds the protein beta-arrestin (5). The
beta-arrestin prevents prevents the receptor from activating more G proteins, thus giving
rise to an inactive or desensitized receptor.