 The following experiment is a true classic, which helped to establish a
new concept in cell signaling.
The following experiment is a true classic, which helped to establish a
new concept in cell signaling.
 The following experiment is a true classic, which helped to establish a
new concept in cell signaling.
The following experiment is a true classic, which helped to establish a
new concept in cell signaling.
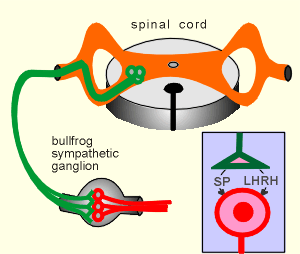
It was conducted with a bullfrog sympathetic post-ganglionic neurons (red in figure to right)
These neurons are regulated by acetylcholine and also by two neuropeptides, namely Substance P (SP) and Luteinizing Hormone-Releasing Hormone, LHRH, (also known as Gonadotropin-Releasing Hormone).
Both neuropeptides work through G(i) to activate K+ channels and thus have an inhibitory working on the neuron.
In the experiment the sympathetic ganglion was removed and the neurons cultured in vitro.
The cultured neurons were then used in electrophysiological experiments.
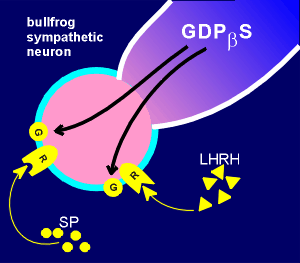 The whole cell
configuration of the patch-clamp was used (review
patch-clamp configurations?)
The whole cell
configuration of the patch-clamp was used (review
patch-clamp configurations?)
This is the configuration where the cell membrane is disrupted, and thus the fluid inside the patch electrode mixes with the cytosol.
In this configuration it is possible to introduce material into the cell by including it in the electrode buffer.
In the experiments with the bullfrog neuron the substance GDPbetaS was introduced.
GDPbetaS is a GDP with a sulfur attached to the beta P group.
It acts as a GTP analogue.
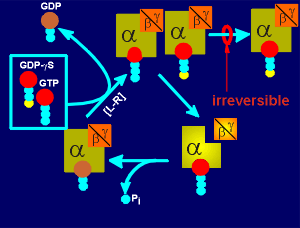 The figure to the right shows what GDPbetaS can do in a
cell.
The figure to the right shows what GDPbetaS can do in a
cell.
When the G protein of a cell is activated (i.e. through a ligand-receptor complex, L-H) then the GDP in the G protein is exchanged for GTP.
GDPbetaS acts as a GTP analogue i.e. the G protein can just as easily pick a GDPbetaS as a normal GTP.
With a normal GTP the G protein then dissociates into the active alpha and beta/gamma subunits and ultimately returns to the inactive GDP bound trimeric form (review movies of the activation and inactivation of G proteins?).
If the G protein has grabbed a GDPbetaS, however, then the G protein becomes locked in an inactive trimeric form (i.e. binding of GDPbetaS is irreversible).
With continued ligand-receptor activation more and more of the G protein will come into the inactive form so that finally the signaling comes to a stand-still because of the lack of any G protein for activation.
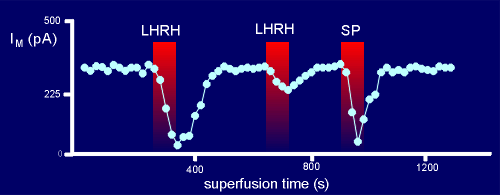 In the experiments with the bull frog neurons, loaded with
GDPbetaS, LHRH was introduced via a superfusion system to the extracellular medium.
In the experiments with the bull frog neurons, loaded with
GDPbetaS, LHRH was introduced via a superfusion system to the extracellular medium.
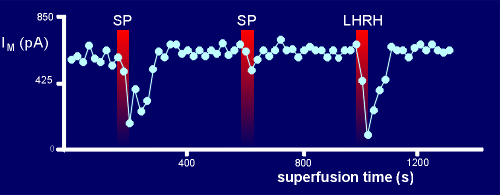
This quickly resulted in a type of K+ current known as an M current.
Thus, the LHRH had activated the K+ channel.
Normally, the LHRH can induce many M currents if given in pulses to the nerve cell preparation.
When a second pulse of LHRH was given to the GDPbetaS treated neuron, however, only a small response was seen.
The logical explanation is that during the first pulse of LHRH there was sufficient G protein to give a response but that during this activation there is a gradual accumulation of the G protein into the inactive GDPbetaS-bound form.
Thus when the second pulse of LHRH arrives there is simply insufficient G protein available to support LHRH signaling to the K+ channel and thus the response is attenuated.
SP however, can still give a strong response, suggesting that the G protein used by SP is sequestered in a different place from the G protein used by LHRH i.e. different signalling domains.
 Support for this comes from experiments where the order
of giving the neuropeptides was reversed.
Support for this comes from experiments where the order
of giving the neuropeptides was reversed.
At the first pulse SP gave a good response (at this point there was a lot of G protein available).
For the second pulse too much of the G protein had been pushed into the GDPbetaS-bound form to give full response.
This had no effect, however, on the response to LHRH because it has its own domain of G protein which is still intact .
While there may be alternative explanations for the above results, certainly the explanation invoking domains seems very plausible.
 Simmons & Matter,
1991. J. Neurosci. 11: 2130-2134
Simmons & Matter,
1991. J. Neurosci. 11: 2130-2134