 The adenylyl
cyclase system is involved in the regulation of diverse processes in the body.
The adenylyl
cyclase system is involved in the regulation of diverse processes in the body.Known G protein effectors (i.e. proteins regulated by G proteins) include:
(1) 2nd messenger producing enzymes (best known are adenylyl cyclase and phospholipase C beta),
(2) ion channels (best established are voltage-operated calcium channels and voltage-operated potassium channels)
(3) kinases (i.e. the receptor kinase beta-adrenergic receptor kinase, beta-ARK or GRK, already discussed)
As mentioned earlier, the existence of multiple effectors is one of the reasons G protein signaling is so versatile.
Adding to the flexibility of G protein mechanisms is the fact hat many of the effectors can be regulated by both an alpha subunit and a beta/gamma subunit of the G proteins.
In this module of the tutorial I will take a closer look at the two most important 2nd messenger generating effectors of G proteins, namely adenylyl cyclase and phospholipase C beta.
 The adenylyl
cyclase system is involved in the regulation of diverse processes in the body.
The adenylyl
cyclase system is involved in the regulation of diverse processes in the body.
This regulation often involves regulating the activity of enzymes within cells.
Use the link to the right to see how receptors can, via adenylyl cyclase and cyclic AMP, regulate enzyme activity and thus cellular programs in liver cells and fat cells.
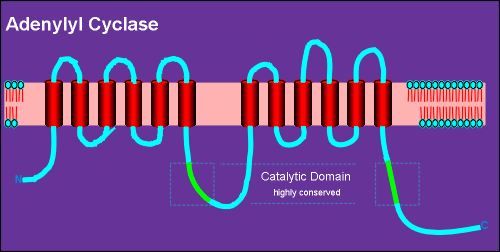 Adenylyl cyclases are
large membrane-bound proteins.
Adenylyl cyclases are
large membrane-bound proteins.
All known adenylyl cyclases consist of an intracellular N-terminal, 6 transmembrane regions, (alpha helixes, indicated in red) a highly conserved catalytic domain (green) and then a repeat of this same basic structure.
Thus it has a total of 12 transmembrane regions.
It is believed that the activated Gs alpha subunit of G proteins binds directly to the catalytic domains, thus activating the enzyme to convert ATP into cyclic AMP.
There is in fact a large family of adenylyl cyclases.... a present there are 9 members.
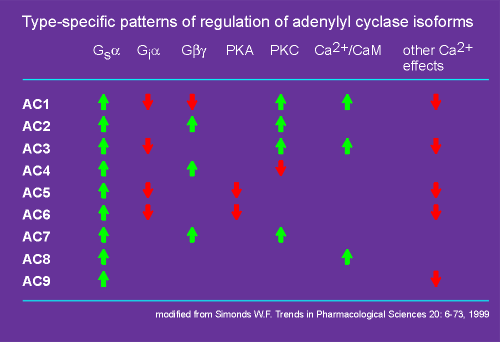 Each isoform of adenylyl cyclase has its own
unique properties (see table to right).
Each isoform of adenylyl cyclase has its own
unique properties (see table to right).
They all are activated by Gs alpha (green arrows), but only some of them are inhibited by Gi alpha (red arrows).
Likewise, only some are regulated by the beta/gamma subunit.
Beta/gamma subunits can give an inhibition or a stimulation depending on which isoforms of the enzyme are considered (i.e. AC1 versus AC2, AC4, AC7).
Some have a negative feedback via inhibition by protein kinase A (PKA, the kinase activated by cyclic AMP).
Also, some can be activated by protein kinase C (PKC), the kinase activated by diacyl glycerol, one of the second messengers produced by the phospholipases (phospholipase beta or gamma). (review phospholipase gamma?)
Thus, there can be "cross-talk" between the adenylyl cyclase and phospholipase signaling systems.
Finally, Ca2+ can affect the activity of some adenylyl cyclases.
Some adenyly cyclase are target proteins for Ca2+-calmodulin, Ca2+/CaM (skip ahead to preview Ca2+-calmodulin?).
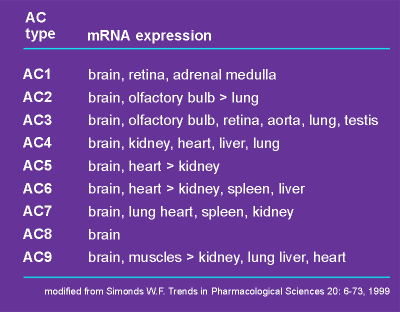 It is also
important to realize that there is tissue specific expression of these isoforms (see table
to left).
It is also
important to realize that there is tissue specific expression of these isoforms (see table
to left).
All adenylyl cyclases have been found in the brain (the brain uses everything it can get to generate signals!).
AC1, AC2 and AC8 are particularly high in the brain.
Which adenylyl cyclase is expressed by a particular cell can have important consequences.
For example, a cell that lacks AC1, AC3, AC5 and AC6 will be nonresponsive to Gi alpha.
If this cell possesses AC2, AC4 or AC7 it may in fact be activated by a Gi protein!
This is because activation of Gi generates beta/gamma subunits that can activate these adenylyl cyclases.
So Gi protein can inhibit cAMP production in one cell type and stimulate it in another!
Thus, it is important which adenylyl cyclase is expressed by a cell in considering the response of the cell to G protein-coupled receptor activation.
 Because adenylyl cyclases are regulated by multiple mechanisms they possess
an integrative capacity.
Because adenylyl cyclases are regulated by multiple mechanisms they possess
an integrative capacity.
They can act as coincidence detectors to detect concurrent stimulation by two or more neurotransmitters.
The idea of coincidence detection has already been introduced in considering the function of the NMDA receptor (review NMDA receptor as a coincidence receptor?).
The link to the right explores a bit further the idea of adenylyl cyclase as a coincidence detector.
 So far you have heard a lot about adenylyl
cyclase and cyclic AMP and very little about guanylyl cyclase and cyclic GMP.
So far you have heard a lot about adenylyl
cyclase and cyclic AMP and very little about guanylyl cyclase and cyclic GMP.
While it was once believed that the guanylyl cyclase signaling system would be similar to the adenylyl cyclase system, surprisingly, it has turned out that they are very different.
In fact guanylyl cyclases are not regulated by G proteins at all but are themselves receptors!
Moreover, there are two quite different types of guanylyl cyclases, in contrast to adenylyl cyclases which are all highly similar isozymes.
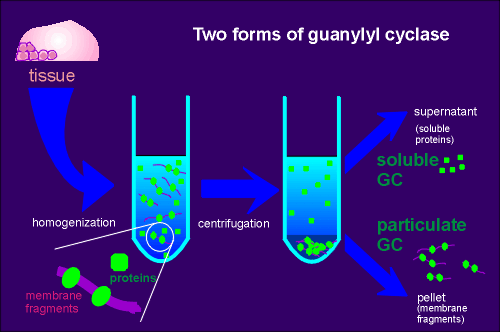
The first hint of two different types of guanylyl cyclases was made in the early 1970's.
It was noted that during extractions of the enzyme from tissue (in an attempt to isolate and purify the enzyme) the guanylyl cyclase activity was found both in the particulate (pellet) and the supernatant fraction of tissue extracts (see figure).
Proteins of the particulate fraction are usually membrane bound proteins whereas proteins found in the supernatant of tissue extracts usually represent soluble cytoplasmic proteins.
Thus came the terminology particulate guanylyl cyclase and soluble guanylyl cyclase.

 In
contrast, adenylyl cyclase activity was found to be only particulate, an
observation in keeping with subsequent research showing all forms of adenylyl cyclase to
be membrane bound proteins.
In
contrast, adenylyl cyclase activity was found to be only particulate, an
observation in keeping with subsequent research showing all forms of adenylyl cyclase to
be membrane bound proteins.
The search for the exact nature of the particulate and soluble guanylyl cyclase took many years.
It was ultimately found that particulate guanylyl cyclase was a membrane bound receptor for a peptide hormone called atrial natriuretic peptide (ANP).
Soluble guanylyl cyclase also proved to be a receptor, not to a peptide but to a gas, nitric oxide (NO).
Use the two links to see an overview of the research that led to the characterization of particulate and soluble guanylyl cyclases.
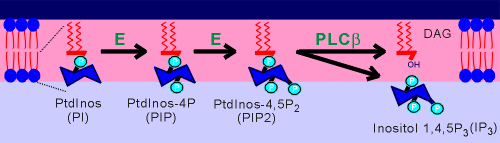 About 30% of the phospholipids of the inner
envelope of the cell membrane are phosphtidyl inositols (PtdInos).
About 30% of the phospholipids of the inner
envelope of the cell membrane are phosphtidyl inositols (PtdInos).
The inositol sugar component of the phospholipid can become phosphorylated by kinases present in the cell membrane to form first PIP and then PIP2.
Early in the study of this signaling system it was thought that one of the kinases would be the receptor-regulated component.
It was soon firmly established, however, that the lipase played this critical function.
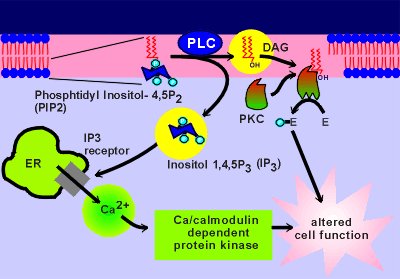 Given to the left is a figure discussed earlier in
relation to phospholipase C gamma (review phospholipase gamma?)
Given to the left is a figure discussed earlier in
relation to phospholipase C gamma (review phospholipase gamma?)
Like PLC gamma, PLC beta also degrades PIP2 to form two second message molecules.
The lipid part of the phospholipid forms diacylglycerol (DAG).
DAG is very hydrophobic and thus stays in the membrane where it binds to and activates a kinase called protein kinase C (PKC).
PKC is located near the plasma membrane and binding of DAG is thought to draw it further into the membrane (kinases are discussed in more detail in the next module of this tutorial).
The phosphorylated sugar component of the phospholipid form IP3, which is water soluble.
Thus, IP3 is free to diffuse into the cytosol in search of its receptor molecule, a Ca2+ channel on the membrane of the endoplasmic reticulum (ER).
To the right is a movie showing IP3 activation of IP3 channels on the ER.
You have seen a similar version of this move when discussing how an influx of Ca2+ can lead to a mobilization of intracellular Ca2+ (review earlier movie?).
The IP3 receptor can be activated by both IP3 and Ca2+.
IP3 has a much longer diffusion distance that Ca2+.
This is why, in the present movie, the source of IP3 (i.e. the PLC on the membrane) can be much further away from the intracellular Ca2+ release channels and still active these channels than was the case for the voltage-operated Ca2+ channels in the earlier movie.
Once the IP3 has activated some receptors (small blue balls that attach to the receptor) the released Ca2+ can activate other IP3 channels and, if the activation is strong enough, a self propagating wave of Ca2+ can be created.
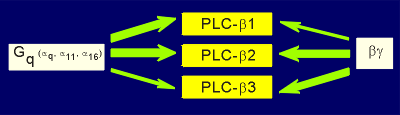 Similar to the situation
with adenylyl cyclases, there are isoforms of phospholipase C beta, although not nearly as
many as with adenylyl cyclase.
Similar to the situation
with adenylyl cyclases, there are isoforms of phospholipase C beta, although not nearly as
many as with adenylyl cyclase.
At present three forms of PLC beta have been found, each with unique signaling characteristics (figure to right).
All are activated by G alpha of the family alpha q and all are activated by beta/gamma.
The beta/gamma subunit works only in an additive fashion with the alpha, and not in a potentiating way, as was the case with some adenylyl cyclases.
The thickness of the green arrows in the above figure indicates the sensitivity of the lipase for activation (e.g. PLC-beta1 is very sensitive to the alpha(q) and less sensitive to beta/gamma activation whereas the opposite is true for PLC-beta3).
In order for the signaling systems to work the second messengers must have short half-lives following their production (otherwise there would be no signaling and kinases etc.would be constantly activated).
Indeed, very efficient systems have evolved for getting rid of the second messengers.
We have earlier discussed how Ca2+, in its evolution as a second messenger, was very effectively removed from the cytosol by pumps, exchangers and Ca2+ binding proteins (review evolution of Ca2+ as second mesenger?)
Equally efficient mechanisms have evolved to get rid of cyclic nucleotides (cyclic AMP and cyclic GMP).
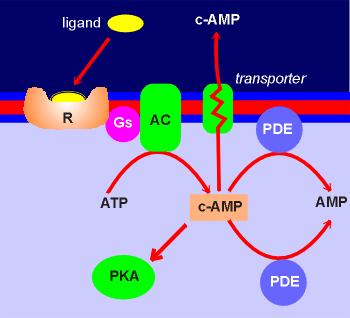 The main mechanism is an enzymatic breakdown of the cyclic
nucleotides.
The main mechanism is an enzymatic breakdown of the cyclic
nucleotides.
The pathway is through enzymes called phosphodiesterases (PDE) which converts cyclic AMP and cyclic GMP to non-cyclic nucleotides.
There are both membrane bound and cytosolic forms of the phosphodiesterases (PDE).
There is also some evidence that transporters which remove cyclic AMP from the cytosol to the extracellular space might play a role in reducing intracellular levels of cyclic AMP.
Cyclic AMP is found extracellularly, for example in the cerebrospinal fluid.
This might reflect the functioning of such a system in cells of the central nervous system.
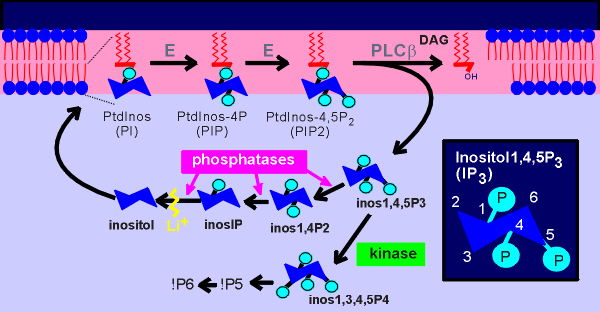 Concerning the second messengers generated by
phopholipases, the IP3 also has a very short half-life in the cytosol.
Concerning the second messengers generated by
phopholipases, the IP3 also has a very short half-life in the cytosol.
IP3 is recycled to inositol via a series of cytosolic phosphatase enzymes.
The inositol generated can then be utilized in the production of new phosphatidyl inosoitol (PtdInos).
As well, IP3 can also be a substrate for a kinase that produces IP4, which has been proposed to be an intracellualar second messenger.
IP4 can be further phosphorylated to generate IP5 and IP6.
Ultimately all inositol phosphates are dephosphorylated to inositol, the latter of which is utilized in the synthesis of new phospholipids.
The monovalent cation lithium (Li+) has been found to inhibit the enzyme inositol monophosphatase and thus blocks the formation of inositol.
Lithium has become an important tool in testing if a signal system is working through inositol phosphates (Li+ blocks recycling of inositol which is essential for the resynthesis of PtdInos thus blocking signaling through phospholipase C).
Lithium is used in the treatment of manic depression as a mood stabilizer .
In the "inositol theory" of manic depression it has been suggested that Li+ acts on overactive inositol signaling systems in brain.
This would explain why lithium has little effect on normal behavior but powerfull effects in manic depression.
***
Finally we come to diacylglycerol (DAG).
This also undergoes a rapid enzymatic recycling to participate in the formation of new phophatidylinositol in the membrane.