 The
discovery that the gas nitric oxide is a biological message molecule was made in research
concerning how the autonomic nervous system regulates blood vessels.
The
discovery that the gas nitric oxide is a biological message molecule was made in research
concerning how the autonomic nervous system regulates blood vessels. 
 The
discovery that the gas nitric oxide is a biological message molecule was made in research
concerning how the autonomic nervous system regulates blood vessels.
The
discovery that the gas nitric oxide is a biological message molecule was made in research
concerning how the autonomic nervous system regulates blood vessels.
This work led to the Nobel Prize for Medicine & Physiology in 1998 (use link to left to see the Nobel press release for this event).
It was known that acetylcholine released by the parasympathetic nervous system causes the relaxation of smooth muscles around blood vessels, thus causing a lowering of blood pressure (i.e same effect on blood pressure as ANP).
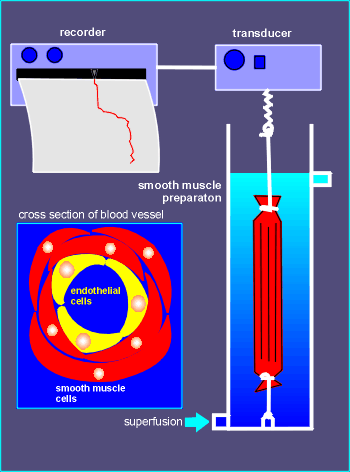
The contraction and relaxation of smooth muscles were analyzed in experimental setups such as the one depicted to the right.
Acetylcholine could be introduced to the tissue via the superfusion medium that was pumped through the system.
In these experiments the neurotransmitter would quickly induce a relaxation of the muscle, as detected by the transducer and passed on to the recorder (see control recording below).
It was assumed that acetylcholine was acting directly on the smooth muscle cells themselves to induce relaxation.
It was possible to "strip-out" the endothelial cells from the smooth muscles prior to placing the preparation in the experimental setup (the reason this was originally done remains obscure).
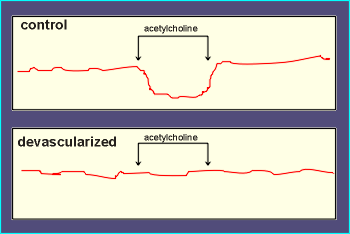 This led to the
surprising finding that acetylcholine was no longer effective in inducing relaxation (see
results for the "stripped" or devascularized tissue to the left).
This led to the
surprising finding that acetylcholine was no longer effective in inducing relaxation (see
results for the "stripped" or devascularized tissue to the left).
From these types of experiments the idea evolved that acetylcholine must be acting on the endothelial cells to release a substance which in turn caused relaxation of the smooth muscles.
This substance was given the name "endothelial-derived relaxation factor", or EDRF.
The characterization of EDRF was a "hot topic" for a number of years, not surprising in view of its blood pressure-lowering effects (thus of economic interest to develop new pharmacons).
The studies leading to the characterization of EDRF (and the Nobel Prize) proved to be very difficult because EDRF was no ordinary signaling substance.
![]() It proved to be a gas, nitric oxide (NO), a well-known
toxic air pollutant!
It proved to be a gas, nitric oxide (NO), a well-known
toxic air pollutant!
 It is not stored in the
endothelial cells but is rather produced on demand (perhaps not surprising as it would be
very difficult for a cell to store a gas).
It is not stored in the
endothelial cells but is rather produced on demand (perhaps not surprising as it would be
very difficult for a cell to store a gas).
This is one of the reasons it was so difficult to characterize.
Once released, the NO is extremely unstable, being easily oxidized (by substance in biological fluids called superoxides) or scavenged by hemoglobin (it binds to the heme group of hemglobin).
This too made characterization of EDRF as NO very difficult.
 The fact that it had a high affinity to hemoglobin gave a
hint as to its ultimate target in the smooth muscles.
The fact that it had a high affinity to hemoglobin gave a
hint as to its ultimate target in the smooth muscles.
In the late 1980s a heme-containing guanylyl cyclase, the so-called soluble guanylyl cyclase, had been characterized.
Indeed, NO was found to bind to the heme group of this cyclase and, in doing so, activate the guanylyl cyclase activity of this enzyme.
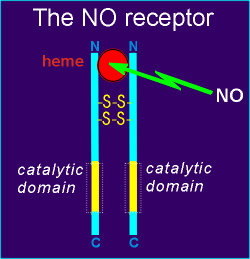
Soluble guanylyl cyclase proved to be the NO receptor.
The receptor is a dimmer of two identical proteins, held together by two disulfide bridges.
The guanylyl cyclase catalytic domains are in the C-terminal, and a binding site for a heme group is formed by the two peptide chains in the N-terminal region.
The entire pathway for acetylcholine regulation of blood has now been worked out.
Given below is a short account of how acetylcholine regulates blood pressure through vasodilatation.
Follow the sequence of events leading to smooth muscle relaxation around blood vessels in the diagram below.
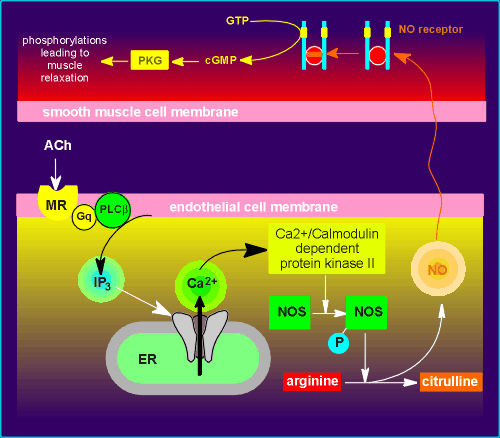 (1) Acetylcholine
(ACh) binds to the so-called muscarinic receptor (MR) on the membrane of the endothelial
cell.
(1) Acetylcholine
(ACh) binds to the so-called muscarinic receptor (MR) on the membrane of the endothelial
cell.
(2) The transmitter-receptor complex activates a Gq protein which in turn activates the enzyme phospholipase beta to generate inositol triphosphate (IP3).
(3) IP3 activates IP3 receptors (a ligand-operated Ca2+ channel on membrane of the endoplasmic reticulum, ER) causing the release of Ca2+ to the cytoplasm.
(4) The released Ca2+ bind to the Ca2+ binding protein calmodulin and Ca2+-calmodulin in turn activates a kinase (Ca2+/Calmodulin dependent protein kinase II).
(5) The kinase phosphorylates the enzyme responsible for the production of NO, an enzyme called nitric oxide synthase (NOS).
(6) Activated NOS produces NO gas in a very complex pathway involving the oxidation of the amino acid arginine and producing NO gas and citrulline.
(7) The NO gas diffuses out of the endothelial cell (it can easily cross membranes) and enters the smooth muscle cell.
(8) The NO binds to the heme group in the NO receptor molecule, activating the catalytic domains of the receptor to produce cyclic GMP.
(9) cyclic GMP activates Protein Kinase G (PKG) which then phosphorylates various proteins in the smooth muscle leading to muscle relaxation.
Nitroglycerin, which has been used for years to lower blood pressure in heart patients, quickly generates NO gas.
Shortly after the discovery of NO and NOS in endothelial cells it was discovered that both substances are also found in the brain.
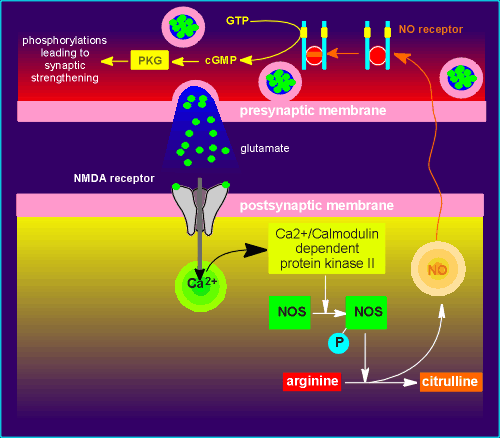 There is some evidence
that this gas is involved in synaptic plasticity (changing the strength of synapses).
There is some evidence
that this gas is involved in synaptic plasticity (changing the strength of synapses).
One model has NO as a so-called retrograde message.
This model is very similar to the mechanism described above for endothelial cell regulation of the smooth muscle.
It involves the NMDA receptor, one of the receptors for the neurotransmitter glutamic acid (review NMDA receptor?)
Glutamate released from the presynaptic nerve terminal activates the NMDA receptor.
From here on, it is identical to the events described earlier for the blood vessel.
The proteins phosphorylated by the PKG have not been identified.
It is believed that NO will be a neurotransmitter of major importance to brain function.
It follows that its receptor, soluable guanylyl cyclase, will also be a major player in brain processes.
 Finally,
if you want to see how scientists have bred ferocious rodents
that rape and kill then you should take a peek at the
article in the December 4, 1995 issue of Time Magazine.
Finally,
if you want to see how scientists have bred ferocious rodents
that rape and kill then you should take a peek at the
article in the December 4, 1995 issue of Time Magazine.
 Use the
link to the right to have a look at what happens when your NOS is knocked out.
Use the
link to the right to have a look at what happens when your NOS is knocked out.