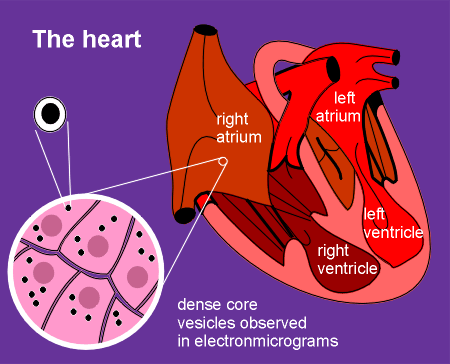
 This story starts with an early
observation that cells in the atrium of the heart possess dense core granules.
This story starts with an early
observation that cells in the atrium of the heart possess dense core granules. This story starts with an early
observation that cells in the atrium of the heart possess dense core granules.
This story starts with an early
observation that cells in the atrium of the heart possess dense core granules.
Dense core granules are usually indicative of peptide secreting cells, leading to an hypothesis that the heart is an endocrine gland.
In 1981 it was discovered that injection of atrial extracts into the rat caused a massive natriuresis (loss of sodium via the kidney) and an accompanying diuresis (loss of water).
Consequently, there was a drop in blood volume and blood pressure.
Injections of extracts from isolated artial dense core vesicles had the same effect.
The substance was give the name atrial natriuretic factor (ANF).
It was hypothesized that ANF was a hormone, produced by the heart, that was involved in the regulation of blood pressure.
With the discovery of the natriuretic activity there began the laborious task of isolating and purifying ANF to determine exactly what it was.
In 1984 the sequence of Atrial Natriuretic Peptide (ANP) was published.
With the structure of the ligand known, the next step was to go after the receptor for ANP.
Molecular approaches were used in this effort and culminated in 1987 with the full sequence information for the rat ANP receptor.
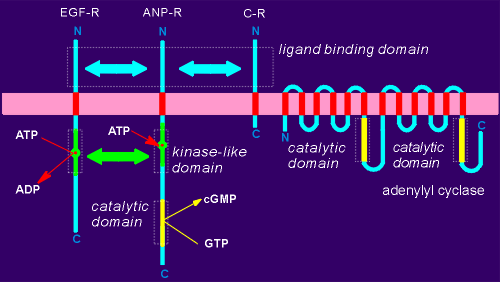 It proved to be a membrane-bound guanylyl cyclase,
i.e. it was the particulate guanylyl cyclase!
It proved to be a membrane-bound guanylyl cyclase,
i.e. it was the particulate guanylyl cyclase!
More surprises were in store with the sequencing of the ANP receptor.
It had a structure very reminiscent of tyrosine receptor kinases (the general structure of epidermal growth factor receptor EGF-R, has been included in the figure to the left).
Homology was found, first, in the ligand binding domains.
Moreover, ANP-R had a pseudo-kinase domain (high homology to the kinase domains of tyrosine kinase receptors but lacking any kinase activity).
Within this kinase-like domain of ANP-R there is even an ATP binding site.
Because of these homologies the ANP-R is considered a distant relative of the tyrosine kinase receptors.
In the C terminal region of the ANP-R however, there is a domain unique to this receptor, namely the guanylyl cyclase domain.
This domain has, as one might expect, a high degree of homology with the cyclase domains of adenylyl cyclase.
A second potential receptor for ANP has also been discovered.
This receptor has a high homology in the extracellular ligand binding region but has a very short C-terminal region, lacking both the kinase-like domain and the cGMP catalytic domain.
The receptor is devoid of any known signaling function.
It has been called the "clearance receptor" (C-R) with the idea that it may function to clear ANP from the blood by providing a high-affinity binding site for the hormone.
There is no experimental evidence that C-R has a biologically important clearance function.
ANP and a closely related peptide, brain natriuretic peptide (BNP), have also been found in the brain.
Injection of ANP (or BNP) in the ventricles of the brain (thus across the blood-brain barrier so that central effects of the peptides can be examined) was accompanied by a loss in salt appetite.
This is an example (one of the very few) where there appears to be a relationship or coordinated function between the action of a peptide in the periphery and its central activity.