 GABA
stands for Gamma aminobutyric acid.
GABA
stands for Gamma aminobutyric acid. GABA
stands for Gamma aminobutyric acid.
GABA
stands for Gamma aminobutyric acid.
GABA is the most important inhibitory neurotransmitter of the brain.
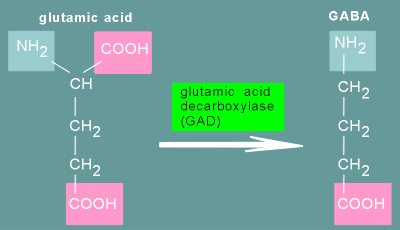
It is synthesized, in a single step, from the amino acid glutamatic acid (= glutamate).
The reaction involves the decarboxylation of glutamate by glutamic acid decarboxylase.
It has been estimated that about 45% of the synapses in the central nervous system (CNS) are GABAergic.
Interestingly, about 25% of CNS synapses have been described as glutaminergic (glutamate as neurotransmitter), with glutamate being the most important stimulatory neurotransmitter in the CNS.
Thus, the combination of gluatame and GABA represents about 70% of the neurotransmission in the CNS.
You will learn more about glutamate receptors in a later module of this tutorial.
Similar to the situation for acetylcholine, with its nicotinic receptor (an ion channel) and muscarinic receptor (a G-protein coupled receptor), GABA has two receptor types.
In fact, without exception, neurotransmitters which possess ion channel receptors also possess G-protein coupled receptors.
It is as if, in evolution, a major investment has been made in the development of a neurotransmitter (i.e. development of enzymes for production of the transmitter and the machinery for releasing it).
Therefore the neurotransmitter will be utilized in as many ways as possible rather than developing new transmitter substances.
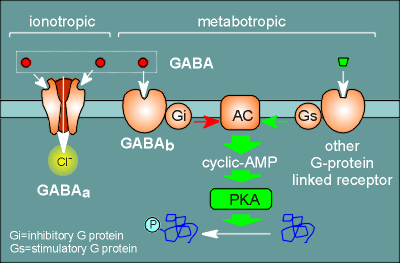 For GABA the two receptor types have been designated the GABAa receptor
and the GABAb receptor.
For GABA the two receptor types have been designated the GABAa receptor
and the GABAb receptor.
The GABAa receptor is an ion channel receptor (a chloride channel) and is the subject matter of this tutorial.
The GABAb receptor is a G-protein coupled receptor.
It couples to the G protein Gi and inhibits adenylyl cyclase (AC) which produces the second messenger cyclic AMP.
In general ion channel receptor mechanisms (referred to as ionotropic) work very fast, while the G-protein coupled mechanisms (referred to as metabotropic) are slower.
Both mechanism are fast to be turned on, ionotropic effects occurring within 20 - 100 microseconds, metabotropic effects occurring within a few milliseconds to seconds.
Metabotropic mechanisms involves activation of kinases (such as the cyclic-AMP dependent kinase PKA) which phosphorylate proteins in bringing about changes.
Therefore the changes they bring about within a cell are long lasting (the proteins must be dephosphorylated by enzymes called phosphatases to reverse the effect of receptor activation).
In contrast, membrane depolarizations or hyperpolarizations brought about by ionotropic mechanisms are very rapidly reversed (the ion imbalance is quickly rectified by ion pumps and ion exchangers).
So, in general, ionotropic mechanisms bring about fast, short duration changes in the cell and metabotropic mechanisms bring about slower, more long lasting changes to the cell.
With the complete sequencing of the subunits of the GABAa receptor through the recombinant DNA approach it was truly remarkable to see how similar this receptor is to the earlier sequenced nicotinic receptor.
Biochemists had already worked out that the GABAa receptor was composed of subunits, 2 alpha subunits and 2 beta subunits with the ligand binding domain in the beta subunits.
The recombinant approach revealed the presence of a new subunit, the gamma subunit, making the GABAa receptor an alpha2, beta2, gamma structure.
The recombinant DNA approach also showed that the overall structure of the subunits of the GABAa receptor were similar to each other and also similar to the earlier characterized nicotinic receptor subunits.
The subunits of the GABAa receptor were found to possess:
 a beta structural loop for ligand binding. (review
nicotinic structural loop?)
a beta structural loop for ligand binding. (review
nicotinic structural loop?)
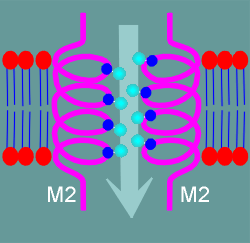 a hydrophilic pore composed from hydroxyl groups of serines and threonines
in the M2 region. (review nicotinic hydrophilic pore?)
a hydrophilic pore composed from hydroxyl groups of serines and threonines
in the M2 region. (review nicotinic hydrophilic pore?)
 a preserved proline in M1 to possibly function as a switch. (review nicotinic switch?)
a preserved proline in M1 to possibly function as a switch. (review nicotinic switch?)
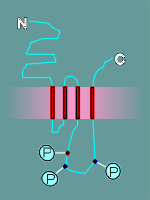 serines and threonines on the intracellular loops to act as regulatory
domains. (review nicotinic regulatory domains?)
serines and threonines on the intracellular loops to act as regulatory
domains. (review nicotinic regulatory domains?)
************************
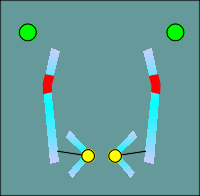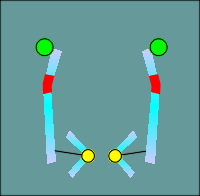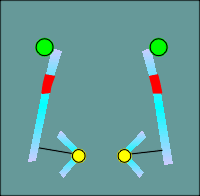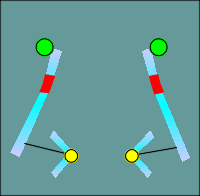
One functional domain that was not the same was the ion filer domain.
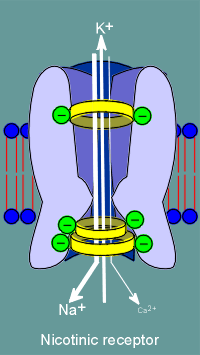
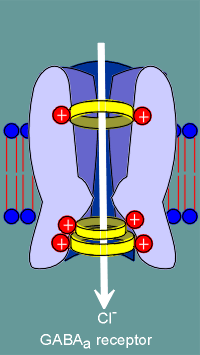 The
molecular biologists noted that some glutamic and aspartic acids present in nicotinic
receptors (location indicated by green balls in figure to left) were changed to lysines
and arginines (red balls) in the GABAa receptor subunits.
The
molecular biologists noted that some glutamic and aspartic acids present in nicotinic
receptors (location indicated by green balls in figure to left) were changed to lysines
and arginines (red balls) in the GABAa receptor subunits.
Thus the idea of ion filters was born, with the positive charges of the lysines and arginines selecting for anions, most importantly the chloride ion.
The negative charges of the glutamic and aspartic acids would select of cations such as Na+ or K+.
This idea has subsequently been substantiated by site directed mutagenesis and expression of the mutated forms of the receptors in Xenopus oocytes.
(review nicotinic ion filter domain?)
The GABAa receptor is truly remarkable for the fact that its action is affected not only by GABA but also by a number of other substances, both endogenous and exogenous.
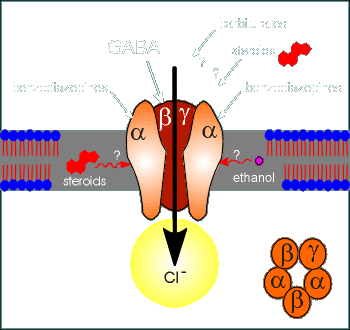 Given to the right is an illustration of some of the
substances know to have an influence on the GABAa receptor.
Given to the right is an illustration of some of the
substances know to have an influence on the GABAa receptor.
First there is GABA, the binding site of which is found in the beta subunits (note, the front beta subunit has been removed in the diagram so you can see the ion channel).
Benzodiazepines have their binding site in the alpha subunit.
The exact binding sites for the barbiturates, steroids and ethanol is not known.
 One idea is that the hydrophobic
steroids, and perhaps ethanol, would have a binding site in the hydrophobic transmembrane
region.
One idea is that the hydrophobic
steroids, and perhaps ethanol, would have a binding site in the hydrophobic transmembrane
region.
Most of these substances are considered neuromodulators of the action of GABA.
This is best illustrated by considering the action of benzodiazepines on the GABAa receptor.
 Benzodiazepines are a class of
pharmacological agents which potentiates the action of GABA on the GABAa receptor.
Benzodiazepines are a class of
pharmacological agents which potentiates the action of GABA on the GABAa receptor.
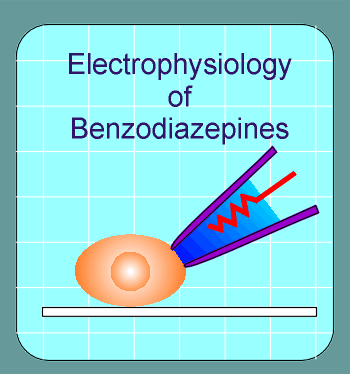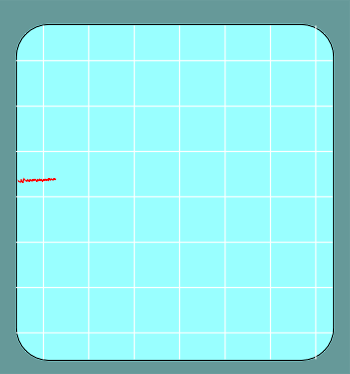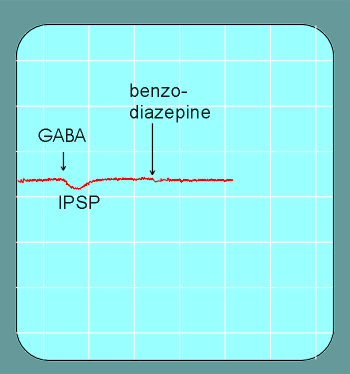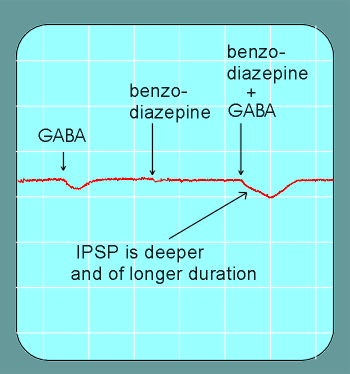
The benzodiazepines alone have little or no effect on postsynaptic inhibitory postsynaptic potentials (IPSPs).
But, when given together with GABA, the IPSPs are deeper and of longer duration as those induced by the same concentration of GABA alone.
 Thus we speak of potentiation.......the benzodiazepine makes
the GABA more potent.
Thus we speak of potentiation.......the benzodiazepine makes
the GABA more potent.
By strengthening the inhibitory pathways the benzodiazepines have a "calming" or sedative effect on the CNS.
They reduce anxiety and, worldwide, they are considered among the most abused of the prescription drugs.
A recent article in the Observer suggests that benzodiazepine may even be under consideration as a weapon of war. (Excerpt from Observer Article).
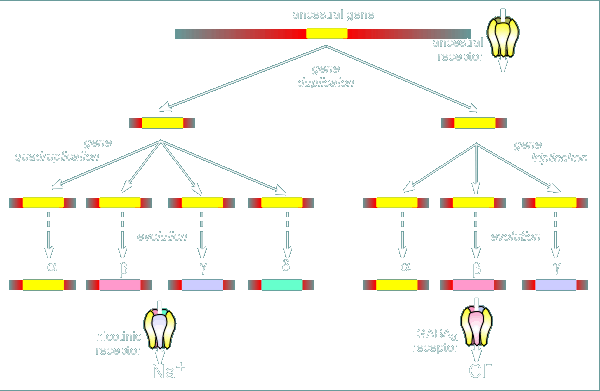 The low but clear sequence homology between the subunits of the nicotinic and
GABAa receptor has suggested that they are evolutionarily related.
The low but clear sequence homology between the subunits of the nicotinic and
GABAa receptor has suggested that they are evolutionarily related.
Thus the gene duplication concept for the evolution of receptors, first introduced when considering the nicotinc receptor (review concept?), is further complicated by the idea that an ancestral gene duplication might be responsible for ultimately giving rise to both nicotinic receptors and GABAa receptors.
In this scenario, one of the duplicates then quadruplicated to give rise to the alpha, beta, gamma and delta subunits of the nicotinic receptor.
The other duplicate triplicated to give rise to the alpha, beta and gamma subunit of the GABAa receptor.
________________
So far, so good......but....
There is, in fact, a family of GABAa alpha subunits, designated alpha-1, alpha-2, alpha-3 .....all the way up to alpha-6.
Which means that the gene for the alpha subunit must have duplicated 6 times to give rise to six genes.
The gene for the alpha subunit is not the only one to have duplicated itself.
......there is a family of GABAa beta subunits, beta-1, beta-2 and beta-3.
......and a family of GABAa gamma subunits, gamma1, gamma-2 and gamma-3.
......and a delta subunit has recently been discovered!
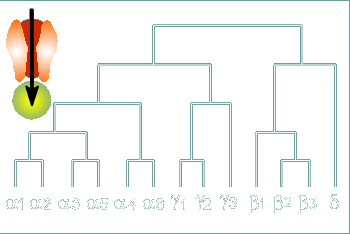 Thus our scheme for the evolution of the receptor becomes more
complicated.
Thus our scheme for the evolution of the receptor becomes more
complicated.
Given to the left is an evolutionary scheme for the various subunits of the human GABAa receptor based on their sequence homologies.
The alpha and gamma subunits show higher homology to each other than to the beta or delta.
Likewise, the beta subunits show high sequence homology with the delta subunit.
The presence of so many different subunits means means that there are potentially many different GABAa receptors.
The selection of at least 1 alpha, 1 beta, and 1 gamma subunit from the repertoire of 13 subunits outlined above allows for the possible existence of more than 10,000 pentametric subunit combinations!
Do they all exist? probably not.....but if just 0.1% exist then we are talking about the brain working through 10 different GABAa receptors!
Researchers are now busy trying to determine the real, rather than the theoretical diversity of the GABAa receptor.
The main approach is to map the brain using antibodies specific to the various subunits.
What is emerging from this research is that the brain is a mosaic of different GABAa receptors, some subunit combinations being high in one brain area and low in another.
The number of major subtypes is probably about 10.
Is there any functional importance to the fact that there are multiple GABAa receptors.
The answer is yes.
To give the most extreme case, while the alpha subunit is the site for benzodiazepine binding, the gamma subunit is required for the benzodiazepines to function well.
 Expression of a GABAa receptor lacking a
gamma subunit means that the receptor is very insensitive to the action of
benzodiazepines.
Expression of a GABAa receptor lacking a
gamma subunit means that the receptor is very insensitive to the action of
benzodiazepines.
This can be nicely observed in the experiments described in the link to the right.
 Researchers are currently trying to
determine more subtle difference between GABAa receptor subtypes and define their
individual roles in brain function.
Researchers are currently trying to
determine more subtle difference between GABAa receptor subtypes and define their
individual roles in brain function.
More details on one such study, conducted by researchers in Amsterdam, can be found with the link to the left.
They show that subunit composition of the GABAa receptor may change under different physiological circumstances and that these changes could have important functional repercussions.
 Another approach being applied in GABAa receptor research is
to immunoprecipitate GABAa receptor subtypes from brain extracts using subunit-specific
antibodies.
Another approach being applied in GABAa receptor research is
to immunoprecipitate GABAa receptor subtypes from brain extracts using subunit-specific
antibodies.
The pharmacological profiles of the isolated receptors are then determined.
Also, molecular approaches are being applied in which different combinations of subunits are brought to expression in transfected cells (similar to the approach described above for the dose-response of the benzodiazepines).
Electrophysiological tests are conducted on the transfected cells to examine the behaviour of the various GABAa receptor subtypes.
These studies have already shown that different recombinant receptor compositions display subtly different properties.... e.g. different sensitivities to GABA and different sensitivities to allosteric modulation by benzodiazepines or neurosteroids.
 A recent study
has also indicated that there may be interesting differences in different GABAa receptors
with respect to the action of kinases on regulatory domains within the subunits.
A recent study
has also indicated that there may be interesting differences in different GABAa receptors
with respect to the action of kinases on regulatory domains within the subunits.
Here the action of different protein kinasese on GABAa function were examined.
Use the link to the left to get more details on these interesting experiments.
These experiments indicate, again, that subunit composition may have important implications to the functioning of the GABAa receptor.
The ultimate goal in this type research is to develop subtype-specific drugs to open the way for novel therapeutic approaches targeting GABAa receptors in the brain.
 This is
the end of the module "the GABAa receptor"
This is
the end of the module "the GABAa receptor"