 It is known that leucine residues in the second
transmembrane (M2) region of the various subunits form a ring (much like the rings of ion
filters discussed earlier).
It is known that leucine residues in the second
transmembrane (M2) region of the various subunits form a ring (much like the rings of ion
filters discussed earlier). It is known that leucine residues in the second
transmembrane (M2) region of the various subunits form a ring (much like the rings of ion
filters discussed earlier).
It is known that leucine residues in the second
transmembrane (M2) region of the various subunits form a ring (much like the rings of ion
filters discussed earlier).
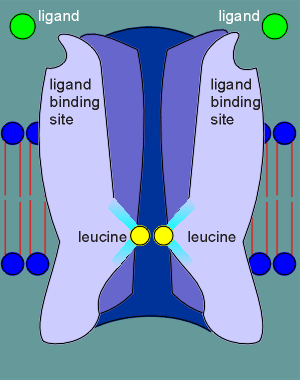
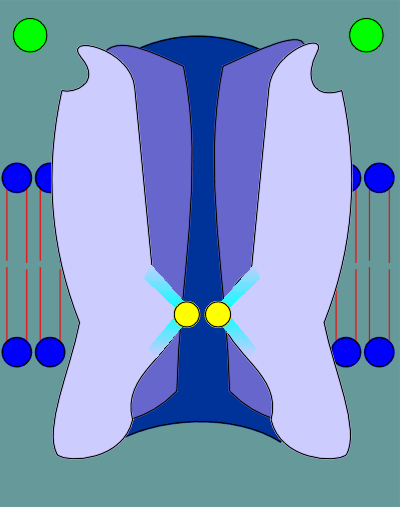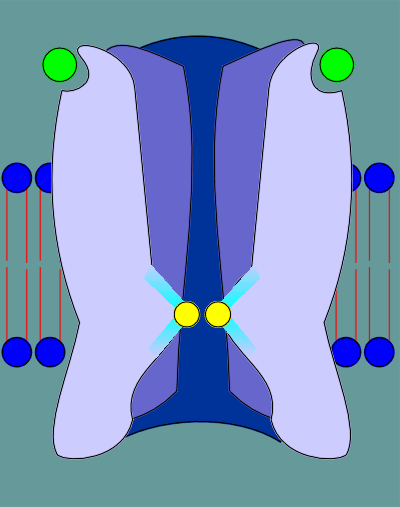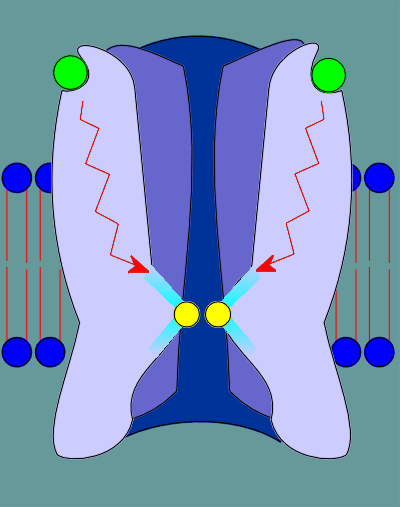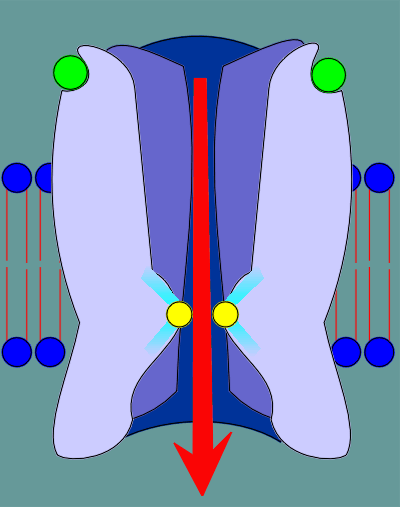
The M2 regions exhibit a kink in their structure such that the leucines are forced into a tight ring that effectively blocks the flow of ions through the ion pore.
The leucines of the two alpha subunits are shown in the cut-away view of the nicotinic receptor in the figure to the right.
 Upon ligand binding
the structure relaxes and consequently the constraints on the leucines are relieved.
Upon ligand binding
the structure relaxes and consequently the constraints on the leucines are relieved.
As a result the tight rings expands as the leucines move back, opening the pore.
With the pore open ions are now free to move into or out of the cell.
To understand how the receptor works it is important to know how the conformational change induced by ligand binding is transduced deep within the receptor where it causes the constraints on the leucines to be lifted.
Some researchers believe that the amino acid proline, found in the first transmembrane region of each subunit, may function as a sort of "switch" which transduces ligand binding into the appropriate response in the ion pore.
The reason they think proline may be involved is because this amino acid possess a very special property which makes it ideal to function as a switch.
This property is outline below.
To understand how proline could function as a switch for the transduction of information, we must first consider peptide structure and the nature of the peptide bond between the amino acids within a peptide.
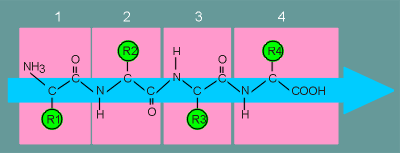 Shown here is a peptide of 4 amino
acids (amino acid 1 is the N-terminal amino acid and amino acid 4 is C-terminal amino
acid).
Shown here is a peptide of 4 amino
acids (amino acid 1 is the N-terminal amino acid and amino acid 4 is C-terminal amino
acid).
Each amino acid has it's own R group (these are the groups that are acetic in amino acids such as aspartic acid, glutamic acid, basic in amino acids such as arginine and lysine, and contain hydroxyl groups in amino acids such as serine and threonine).
The peptide bonds between the amino acids have a structure that leads to a straight peptide chain (indicated by the blue arrow).
Proline has an unusual structure among the amino acids, namely it lacks a free amino group to form the peptide bond.
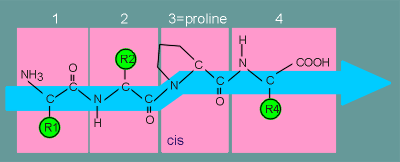 Consequently
in forming a peptide bond it introduces a distortion in the direction of the peptide
chain.
Consequently
in forming a peptide bond it introduces a distortion in the direction of the peptide
chain.
Consider again a peptide of 4 amino acids only in this case the 3rd amino acid is proline.
The proline puts a "kink" in the peptide chain.
For this reason it is unusual to find proline in a transmembrane region of a protein, despite the fact that proline is hydrophobic.
In general, proteins like to go in as direct a manner as possible over the lipid bilayer, i.e. as an alpha-helix, and proline disrupts the helix structure.
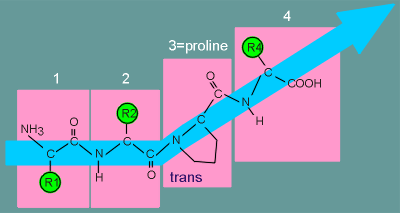 Even
worse, proline can exist in either a "cis" or a "trans" conformation
(the only amino acid which can do so).
Even
worse, proline can exist in either a "cis" or a "trans" conformation
(the only amino acid which can do so).
In the "trans" conformation the direction of the entire peptide chain changes.
This instability make it even very unusual to find in a transmembrane region.
Nonetheless, the various subunits of the nicotinic receptor were shown to possess a proline.
This conservation indicates that the proline must have an important function in the working of the receptor.
Knowing that proline can undergo "cis" to "trans" conformational changes has led to the idea that it could act as the switch.
As such it would transduce the changes brought about by the binding of the ligand into the appropriate response in the ion pore, namely opening of the pore.
 how
might the switch work?
how
might the switch work?The idea is as follows:
The ligands (acetylcholine, indicated as green balls) binds to the ligand binding site in the alpha subunits.
This binding induces a "cis" to "trans" conformational change in the proline, indicated in red.
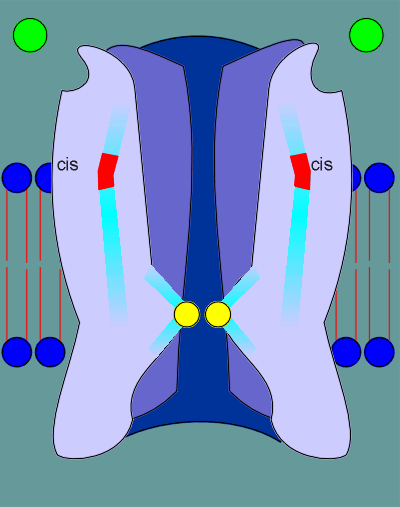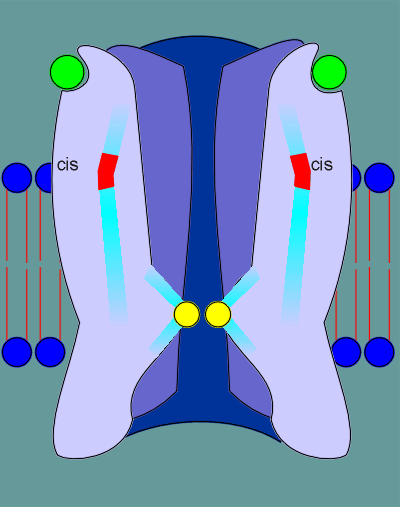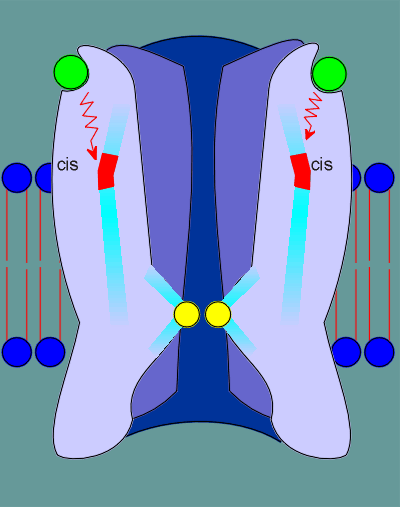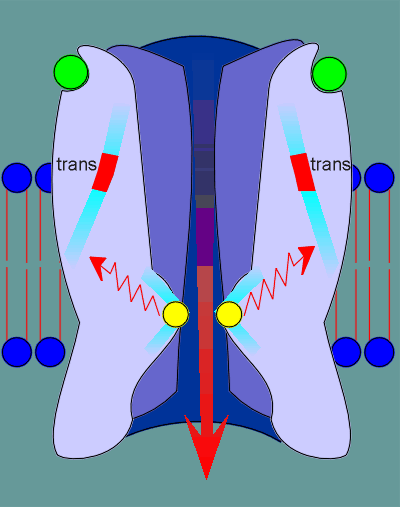
This "cis" to "trans" conformational change causes a restructuring deep within the receptor.
As a consequence of this restructuring constrains that are somehow pushing the leucines into the pore opening are relieved.
Consequently the leucines pull back, the pore opens, and ions are free to flow through the ion pore.
All this occurs within 50 microseconds, from the time that the ligand binds to the receptor to the time the ion pore is fully open.
When the acetylcholine leaves the receptor the proline shifts back to the "cis" conformation and structural constraints are reintroduced to the leucine.
They once again form a tight ring sealing the pore.