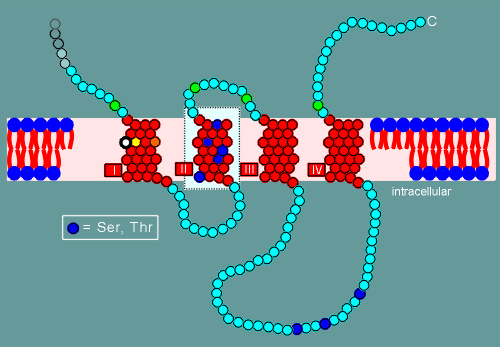
 A very strange phenomenon was noted in
the hyropathy profiles of the nicotinic receptor, namely the presence of a number of
serines (Ser) and threonines (Thr) in the second transmembrane region.
A very strange phenomenon was noted in
the hyropathy profiles of the nicotinic receptor, namely the presence of a number of
serines (Ser) and threonines (Thr) in the second transmembrane region. A very strange phenomenon was noted in
the hyropathy profiles of the nicotinic receptor, namely the presence of a number of
serines (Ser) and threonines (Thr) in the second transmembrane region.
A very strange phenomenon was noted in
the hyropathy profiles of the nicotinic receptor, namely the presence of a number of
serines (Ser) and threonines (Thr) in the second transmembrane region.
This was strange because normally one expects to find only hydrophobic amino acids in the transmembrane regions.
The amino acids serine and threonine possess hydroxyl groups (OH) in their side chains (see diagram below).
The hydroxyl group can form hydrogen bonds and thus the presence of this group in a molecule makes it more hydrophilic.
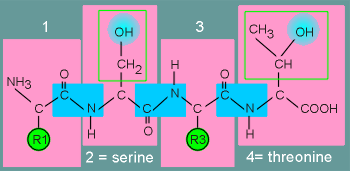 In
the diagram to the left is a peptide composed of 4 amino acids, with serine in position 2
and threonine in position 4 (peptide bonds between the amino acids are indicated in the
blue boxes).
In
the diagram to the left is a peptide composed of 4 amino acids, with serine in position 2
and threonine in position 4 (peptide bonds between the amino acids are indicated in the
blue boxes).
The "R" groups of the various amino acids stick out of the peptide chain.
For serine and threonine the "R" groups contain the OH group.
The presence of these hydrophilic groups in the transmembrane region lead to the idea that, perhaps, they had something to do with the formation of an ion pore, namely a hydrophilic pore.
 In
the diagram given below the serines and threonines are represented by a "ball and
stick" with the hydrophilic hydroxyl group given in light blue (as indicated to the
left)
In
the diagram given below the serines and threonines are represented by a "ball and
stick" with the hydrophilic hydroxyl group given in light blue (as indicated to the
left)
If one was to look down upon a nicotinic receptor in the membrane one would see the five subunits forming a large ring (each subunit is represented by a yellow circle in diagram below).
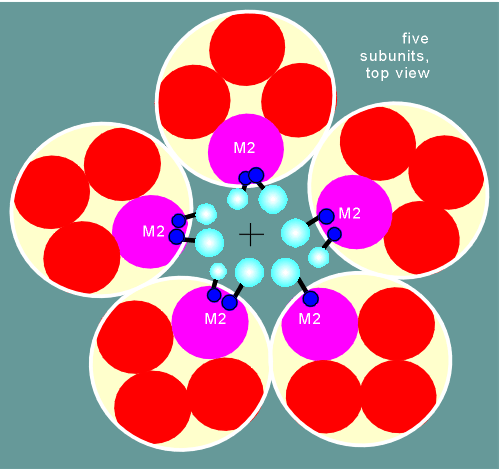 Each circle in
turn would have 4 circles inside, representing the four transmembrane regions (the red and
purple circles).
Each circle in
turn would have 4 circles inside, representing the four transmembrane regions (the red and
purple circles).
To construct a hydrophilic pore the subunits are arranged in such a way so that the second transmembrane region of each subunit comes to lie around the central pore (the M2 purple circles).
Within the M2 transmembrane region are the serines and threonines, with their hydrophilic hydroxyl groups.
To make a hydrophilic pore you simply stick the hydroxyl groups into the pore.
This makes a nice environment for charged ions which are expected to go through the pore.
This construction also solves the problem of having hydrophilic groups in a hydrophobic transmembrane region.
 Rather
than sticking into the hydrophobic lipid bilayer the hydroxyl groups stick into the water
environment of the pore.
Rather
than sticking into the hydrophobic lipid bilayer the hydroxyl groups stick into the water
environment of the pore.
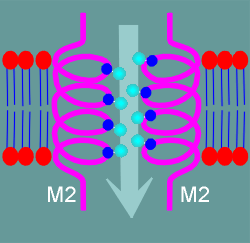
Illustrated to the left is a side view of two of the M2 alpha helices.
The periodic serines and threonines are located within each helix in such a way that they can stick the hydrophilic OH groups into the watery pore.
A hydrophilic pore, rather than a charged pore, would function equally good for anions or cations.