the ligand binding domain
 Classical biochemists were busy for a number of
years prior to the work of Numa, characterizing the ligand binding domain of the nicotinic
receptor (using radioactive acetylcholine to identify fragments of the receptor which bind
the ligand and then sequencing the fragments)
Classical biochemists were busy for a number of
years prior to the work of Numa, characterizing the ligand binding domain of the nicotinic
receptor (using radioactive acetylcholine to identify fragments of the receptor which bind
the ligand and then sequencing the fragments)
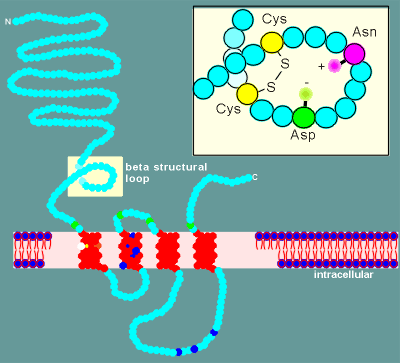
The ligand binding site was found to possess a disulfide
bridge between cysteine residues (Cys) which hold the structure in what is called a beta
structural loop.
Once the whole receptor has been sequenced through the
recombinant cDNA approach, the ligand binding structure characterized by the biochemists
could be located in the extracellular region of the alpha subunit (see diagram).
The idea, formed first by the biochemists, is that the
disulfide bridge forms a sort of "pocket" in which the ligand can sit.
Within the pocket are amino acids with side chains with
which the ligand can non-covalently bind, and thus induce a change in the structure of the
receptor.
For the nicotinic receptor the amino acid asparagine (Asn)
and aspartic acid (Asp) are within the beta structural loop.
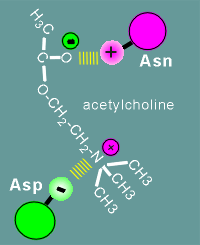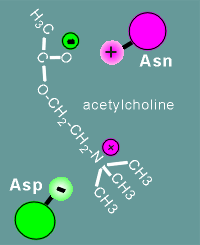 asparagine possesses a side group
with a "+" charge and aspartic acid possesses a side group with a "-"
charge.
asparagine possesses a side group
with a "+" charge and aspartic acid possesses a side group with a "-"
charge.
Acetylcholine also has a "+" and "-"
charge.
The "+" and "-" charges of the
acetylcholine and those of the amino acids of the receptor form a ion pairs, and thus a
high affinity site is created for the ligand.
This type of ligand binding site, where disulfide bridges
form pockets for ligand binding, has been found in many different types of receptors.
Similar functional domains have been found in tyrosine
kinase receptors and in G-protein coupled receptors.
So the principle of the pocket with reactive side chains for
ligand binding seems to be a fairly general one for receptor ligand interactions.
 Classical biochemists were busy for a number of
years prior to the work of Numa, characterizing the ligand binding domain of the nicotinic
receptor (using radioactive acetylcholine to identify fragments of the receptor which bind
the ligand and then sequencing the fragments)
Classical biochemists were busy for a number of
years prior to the work of Numa, characterizing the ligand binding domain of the nicotinic
receptor (using radioactive acetylcholine to identify fragments of the receptor which bind
the ligand and then sequencing the fragments) asparagine possesses a side group
with a "+" charge and aspartic acid possesses a side group with a "-"
charge.
asparagine possesses a side group
with a "+" charge and aspartic acid possesses a side group with a "-"
charge.