 The first receptor for
neurotrophins was discovered as an oncogene.
The first receptor for
neurotrophins was discovered as an oncogene.There is a large family of trophic factors which activate tyrosine kinases receptors in the central nervous system.
 The first receptor for
neurotrophins was discovered as an oncogene.
The first receptor for
neurotrophins was discovered as an oncogene.
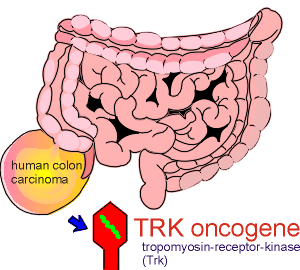
It was initially identified in a human colon carcinoma.
Molecular characterization of the oncogene found in this carcinoma showed that it coded for a what appeared to be a receptor kinase.
It was given the name tropomyosin-receptor-kinase or Trk (pronounced track).
Although the original discovery was made in the colon, the Trk proto-oncogene product was, surprisingly, subsequently shown to be primarily restricted to neural tissue.
Further research showed that the proto-oncogene represented a tyrosine kinase receptor for a neurotrophin.
Neurotrophins are large proteins involved in stimulating the growth and differentiation of the central nervous system during development.

 While
the developmental topic is beyond the scope of this tutorial, the link to the left
illustrates a recent study demonstrating a role for neurotrophins in nerve growth.
While
the developmental topic is beyond the scope of this tutorial, the link to the left
illustrates a recent study demonstrating a role for neurotrophins in nerve growth.
On the right is a link giving access to some interesting findings with gene knock-outs mice which indicated the importance of neurotrophins to brain development.
It is becoming increasingly apparent that for a number of tissues, including brain tissue, tyrosine phosphorylations are important in the adult animal.
It is this topic that will be persued in this tutorial module.
Postmitotic neurons have been shown to express high levels of tyrosine kinase activity, indicating a role for tyrosine phosphorylation in the function of the mature brain.
The most common neurotrophins are nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF).
Research has shown that there is a family of tyrosine kinase receptors in the brain.
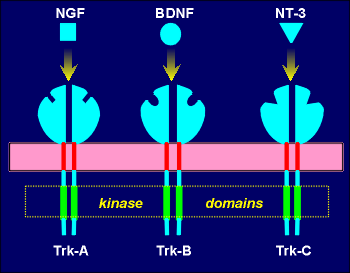 The receptors have been called Trk receptors, to reflect
their initial discovery as the oncogenic product of the Trk oncogene.
The receptors have been called Trk receptors, to reflect
their initial discovery as the oncogenic product of the Trk oncogene.
The first Trk proto-oncogene to be discovered, designated Trk-A, proved to be the receptor for NGF.
Trk-B was found to be the receptor for BDNF and a third Trk receptor, Trk-C, proved to be the receptor for a new neurotrophin, neurotropin-3 (NT-3).
The Trk receptors, like all tyrosine kinase receptors, have large extracellular ligand binding domains, single transmembrane domains and intracellular kinase domains.
Activation of the receptors requires ligands-induced dimerization of the receptor.
Dimerization is followed by transmolecular phosphorylation of intracellular tryrosines of the receptors.
This yields an active receptor complex which then phosphorylates various intracellular proteins to bring about changes in the cell.
The amounts of mRNA for NGF and BDNF found in neurons, in general, has been found to reflect the activity of the neurons (studies performed mostly in rat hippocampus and cerebral cortex).
Up-regulation (more mRNA) is mediated by glutamate via both NMDA receptors and non-NMDA receptors and down regulation is mediated primarily by GABA through a GABAa receptor.
It has been found that these regulatory mechanisms are involved in the maintenance of normal physiological amounts of NGF and BDNF.
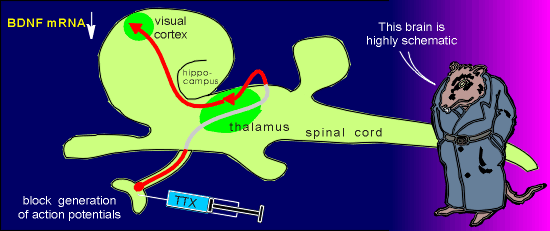 As an example of the
activity-dependence of neurotrophin gene expression, consider input to the visual cortex.
As an example of the
activity-dependence of neurotrophin gene expression, consider input to the visual cortex.
Injection of tetrodotoxin (TTX) into the eye results in a rapid down-regulation in BDNF mRNA in the visual cortex.
The TTX is a blocker of voltage-operated Na+ channels and thus it blocks the generation of action potentials.
With TTX treatment the neurons in the eye can not produce action potentials and thus can not activate neurons that ultimately lead to activation of neurons in the visual cortex.
 The same results can be obtained by
placing the animals in continual darkness.... a rapid down-regulation of BDNF.
The same results can be obtained by
placing the animals in continual darkness.... a rapid down-regulation of BDNF.
Upon restoring light the amounts of layer-specific BDNF mRNA in the visual cortex is rapidly restored.
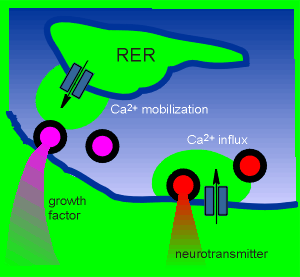
Both NGF and BDNF have been found to be released in a constitutive Ca2+ independent way (a non-regulated pathway) and through a Ca2+ regulated activity-dependent pathway (i.e. a regulated pathway).
 Studies on hippocampal slices and
and primary cultures of hippocampal neurons has shown that regulated secretion does not
involve extracellular Ca2+ (i.e. secretion still occurred when the tissue was cultured in
Ca2+-free medium and treated with glutamate). (review
structure and function of hippocampus?)
Studies on hippocampal slices and
and primary cultures of hippocampal neurons has shown that regulated secretion does not
involve extracellular Ca2+ (i.e. secretion still occurred when the tissue was cultured in
Ca2+-free medium and treated with glutamate). (review
structure and function of hippocampus?)
This indicated that internal stores of Ca2+ are involved in the secretion of the growth factors. (review cellular distribution of Ca2+?).
This idea was confirmed by the observation that treatment with thapsigargin (which causes depletion of Ca2+ stores in the endoplasmic reticulum) caused a drastic reduction in the activity-dependent release of the growth factors.
Thus the release of the growth factors is "unconventional" in comparison to the release of classical neurotransmitters (that depend on extracellular Ca2+ via voltage-operated Ca2+ channels).
Mobilization of intracellular Ca2+ has been found to be involved in supporting secretion of several pituitary gland hormones (interestingly, one of these is growth hormone which has many characteristics in common with the neurotrophins).
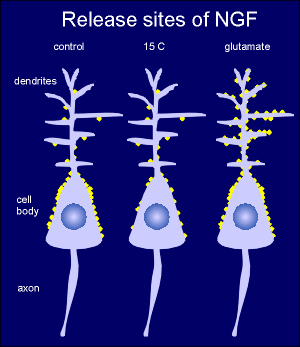 To identify
release sites of neurotrophins high-affinity monoclonal antibodies to NGF were used to
reveal sites of release after depolarization of cultured hippocampal neurons with
glutamate.
To identify
release sites of neurotrophins high-affinity monoclonal antibodies to NGF were used to
reveal sites of release after depolarization of cultured hippocampal neurons with
glutamate.
In control neurons at 37C release sites (yellow circles) were found predominantly around the perikaryon.
Lowering the temperature to 15C, at temperature at which constitutive pathways are largely blocked, eliminated most of this positive reaction.
Thus release at the perikaryon is largely constitutive.
It is thought that this basal unregulated release of neurotropins is required for maintenance of the neuron.
Treating with glutamate (or depolarizing the neuron with K+) increased the signal around the dendrites.
This indicates that this is the site of the Ca2+ dependent regulated secretion of NFG.
The compartment for constitutive neurotrophin release in neuron remains to be established but it is thought that it will be via small transfer vesicles from the RER.
For regulated release, secrtory granules containing neurotrophin immunoreactive material, has been found in the dentrites.
In vivo and in vitro studies have shown that neurotrophins can enhance the release of neurotransmitters.
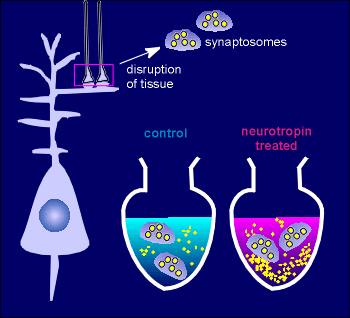 For example, in synaptosomal preparations of the hippocampus neurotropins,
such as BDNF, enhanced the release of glutamate.
For example, in synaptosomal preparations of the hippocampus neurotropins,
such as BDNF, enhanced the release of glutamate.
Synaptosomes are nerve terminals that have been prepared by disrupting nervous tissue.
They contain the synaptic vesicles (large yellow circles) and if depolarized (usually through adding high K+ solution) they release neurotransmitter (small yellow circles in medium).
While the BDNF alone had little effect on the release of neurotransmitter, it enhanced the release in response to depolarization.
Presumably, there is Trk receptors on the presynaptic terminals, activation of which sensitizes the neurotransmitter secretory machinery to depolarization.
In vivo it has been found that injection of neurotrophins into the hippocampus immediately enhanced electrical activity associated with neurotransmitter release.
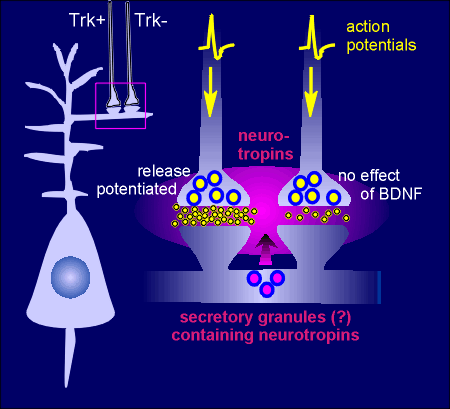
There is mounting evidence that neurotrophins may act as "retrograde" messengers in the brain.
The idea is that there would be an activity-dependent release of neurotrophins from post-synaptic neurons.
The released neurotrophin would act on presynaptic Trk receptors to increase secretion of neurotransmitter in response to depolarization (e.g. the presynaptic neuron possessing Trk receptors indicated by Trk+ in the figure to the right).
Thus, when action potentials reach the terminal the response is greater release of transmitter, indicated by yellow circles in the synapse in the figure to the right.
The neurotrophins by themselves do not induce neurotransmitter release but would increase release in response to the action potential.
Presynaptic terminals lacking Trk receptors (the Trk- neuron in figure) would display no such effect.
 How might the neurotrophins do
this? ......presumably through phosphorylation of a presynaptic protein or proteins.
How might the neurotrophins do
this? ......presumably through phosphorylation of a presynaptic protein or proteins.
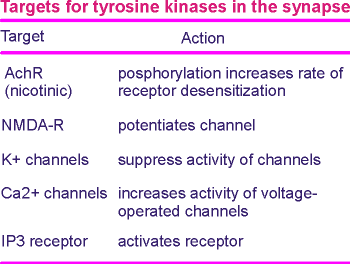
A good candidate is Ca2+ channels or proteins associated with Ca2+ signaling.
Indeed it has been found that voltage-operated Ca2+ channels are a target for Tkr receptors (table to the left).
Activation of tyrosine kinases has been shown to increase activity of the channels.
Thus the phosphorylated voltage-operated Ca2+ channels associated with the exocytotic machinery is more sensitive to to depolarizations and, as a result, secretion is potentiated with neurotrophin treatment.
As the table above indicates, the action of neurotrophins have the potential to go beyond action on pre-synaptic Ca2+ channels.
Nicotinic receptors, NMDA receptors, K+ channels and IP3 receptors have all been shown to be potential targets for the neurotrophins and their Trk receptors.
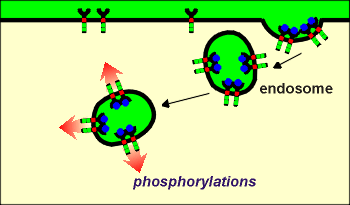 As discussed earlier (review earlier discussion?),
and illustrated to the right, tyrosine kinase receptors such as the Trk receptors for the
neurotrophins often undergo endocytosis following binding of ligands.
As discussed earlier (review earlier discussion?),
and illustrated to the right, tyrosine kinase receptors such as the Trk receptors for the
neurotrophins often undergo endocytosis following binding of ligands.
One idea concerning this endocytosis is that it would bring activated kinases into the cell.
The catalytic domains would be directed into the cytosol from the endosome.
These endosomes could then carry the activated kinases deep into the cell or, in the case of a nerve terminal, into deeper parts of the terminal.
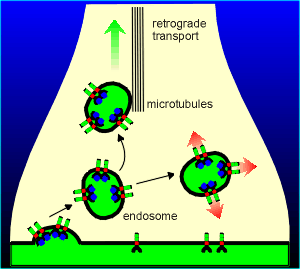 An possibility that is gaining more
and more experimental support is that these endosome complexes could undergo retrograde
transport via transport mechanisms on the the micortubules.
An possibility that is gaining more
and more experimental support is that these endosome complexes could undergo retrograde
transport via transport mechanisms on the the micortubules.
In this way the active kinase could find its way to the cell body of the neuron.
In the cell body the kinases could then be involved in the phosphorylation of transcription factors leading to the expression of specific genes.
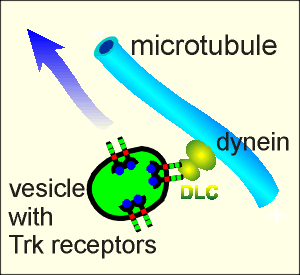 Recent
support for this has been found it the discovery that the C-terminal region of Trk
receptors bind dynein, a retrograde transport protein.
Recent
support for this has been found it the discovery that the C-terminal region of Trk
receptors bind dynein, a retrograde transport protein.
The receptors bind to the Dynein Light Chain (DLC) subunit of dynein (figure to right).
The function of DLC is to bind in a specific way with cargo proteins for transport in the retrograde direction.
The discovery that DLC binds with Trk receptors makes it highly likely that the Trk containing endosomes represent a cargo complex for retrograde transport.
It has long been the dream of clinical neurologists to someday replace dysfunctional brain cells, such as in neurological disorders like Parkinson's disease or Alzheimer's disease, or in cases where neurons have been damaged or destroyed (e.g. in strokes or in accidents).
The realization of this dream has taken a major step forward in the 1990's with the discovery of stem cells.
Stem cell are undifferentiated cells which, if given the proper signals, can differentiate into a number of different cells such as neurons, glial cells, blood cells or muscle cells.
Stem cells are present in adult animals (even in the brains of adults!) but are more plentiful in embryos, where they are referred to as embryonic stem cells.
 Stem cell research took such tremendous strides in the 1990's that in
December 1999 Science magazine declared them to be "The cell of the Decade".
Stem cell research took such tremendous strides in the 1990's that in
December 1999 Science magazine declared them to be "The cell of the Decade".
The trick for ultimate clinical applications of stem cells is to give them the proper signals to develop the proper cell, e.g. perhaps to produce dopaminergic neurons for transplantation into Parkinson's patients.
In this endeavor it is believed that growth factors and neurotrophins will play a major role in giving the appropriate signals.
Use the link to the right to learn more about this exciting area of research.
Neurotrophins, and their associated tyrosine kinase Trk receptors, have important functions, both in the developing brain and in the functioning of the adult brain.
The neurotrophins are likely involved in the maintenance of neurons, possibly through constitutive release in the region of the cell bodies.
In addition they have clear neurotransmitter function.
These neurotransmitter actions can be on nerve terminals, where membrane bound Trk receptors could phosphorylate voltage operated Ca2+ channels and other proteins important for presynaptic transmitter release.
As well, endocytosed Trk receptors can undergo retrograde transport to ultimately regulate gene expression in the neuron.
These actions make neurotrophins, and tyrosine kinase receptor mechanisms, very important to brain function.