 How to make a knockout mouse
How to make a knockout mouse How to make a knockout mouse
How to make a knockout mouseMolecular biologists have introduced a powerful method to study the function of genes, the so-called gene knock-out.
Before discussing the consequences of gene knockouts I will first give a brief outline of how a knockout is made.
First we must isolate the gene of interest and alter it so it is non-functional.
As an example I will use a recent study where epidermal growth factor receptor (EGFR) knockout mice were produced.
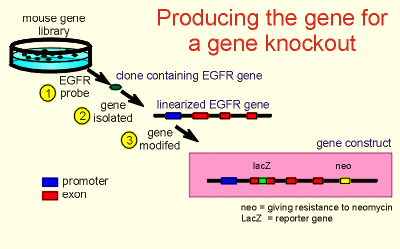 Starting
from a mouse gene library the clone possessing the EGFR gene was identified using a
cDNA probe for EGFR (step 1 in figure to right).
Starting
from a mouse gene library the clone possessing the EGFR gene was identified using a
cDNA probe for EGFR (step 1 in figure to right).
The gene was isolated out of the clone (step 2) and then modified (step 3).
Two modifications were made.
First, the first exon of the gene was modified so that if the gene was to come to expression it would produce a nonfunctional receptor.
The modification made was in fact to introduce the bacterial LacZ gene, which is a so-called reporter gene because expression of LacZ is easy to visualize (it produces a product that is easy to color).
Thus one can easily identify cells expressing the gene construct.
So introducing LacZ has two purposes, it gives a construct which will express a nonfunctional receptor (the most important reason for introducing LacZ) and it gives a marker for anyone who wants to find cells that are trying to express EGFR.
 The second modification was to
introduce the gene "neo" which, if it comes to expression, gives a cell
resistance to neomycin.
The second modification was to
introduce the gene "neo" which, if it comes to expression, gives a cell
resistance to neomycin.
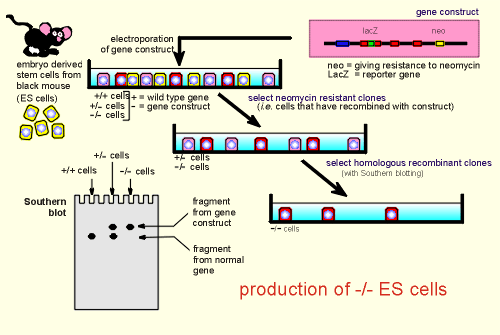
The construct is then introduced into embryonic stem cells (ES cells) by a process called electroporation which made the cells permeable to the construct.
The ES cells are derived from an early non-differentiated embryo (for illustrative purposes from a black mouse).
Following electroporation some cells, in the process of recombination, incorporate the gene construct, occasionally for both alleles.
This means that we end up with a mixture of ES cells......the wild type +/+ (yellow cells in figure), cells containing one copy of the gene construct (+/- cells, indicated in pink), and homologous recombinants in which both alleles are for the introduced gene construct (-/- cells indicated in red).
We get rid of all the wild type cells (which are the vast majority) by selecting those cells that are neuomycin resistant (the neomycin gene was introduced into the construct for this purpose).
What we are left over with are the +/- and the -/- cells.
To identify the -/- cells Southern blots are produced on samples of the cells....the -/- cells have a large fragment because of the introduced LacZ and neo; the +/- cell has both this fragment and the smaller fragment of the wild type allele.
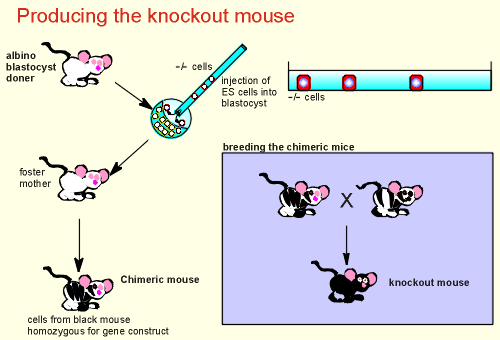 The -/- cells are selected for the
next step, the production of the knockout mouse.
The -/- cells are selected for the
next step, the production of the knockout mouse.
The -/- cells are injected into a blasotcyst (for illustrative purposes from an albino doner), and the embryo implanted into a foster mother.
What we get is a chimeric mouse with some cells +/+ (those derived from the albino doner) and -/- (those derived from the injected ES cells).
The mouse is black and white, the black pigmented regions derived from the injected ES cells, non-pigmented regions derived from cells of the albino doner.
 The entire animal is a mixture of
cells derived from the doner blastocyst and the injected ES cells.
The entire animal is a mixture of
cells derived from the doner blastocyst and the injected ES cells.
The sperm and eggs of the chimeric mice will thus also be a mixture, some cells carrying the + allele and some cells carrying the - allele.
We now start breeding the chimeric mice with the hope that a sperm carrying our gene construct (the - allele) fertilizes an oocyte also carrying our construct.
 In this case we get a homozygous
-/- mouse, our knockout mouse!
In this case we get a homozygous
-/- mouse, our knockout mouse!
In this example this mouse will posses no functional gene for the EGF receptor because the first exon has been modifed...thus we have our EGFR knockout mouse.
A number of mouse knock-out studies have demonstrated that neurotrophin signaling is essential for the development and survival of many different populations of neurons.
The knock-out mice show various degrees of region specific neurodegeneration (i.e which brain area is affected depends on which neurotropin was knocked out).
Sometimes some very surprising results are found with the knock-out approach.
A case in point is the above study of an epidermal growth factor receptor (EGFR) knockout. (Sibilia et al. EMBO J. 17: 719-731, 1998).
Epidermal growth factor, as the name implies, stimulates the division and growth of cells of the skin.
Besides having the expected skin defects, the EGRF knock-out mouse had massive neurodegeneration in frontal cortex, the olfactory bulb, the thalamus and the hippocampus.
Perhaps this is not so surprising when one considers that both skin and brain tissue are derived of ectoderm in the developing embryo.