 The working of a
neuron through ion channels will now be considered to conclude the module "ion
channel receptors".
The working of a
neuron through ion channels will now be considered to conclude the module "ion
channel receptors".  The working of a
neuron through ion channels will now be considered to conclude the module "ion
channel receptors".
The working of a
neuron through ion channels will now be considered to conclude the module "ion
channel receptors".
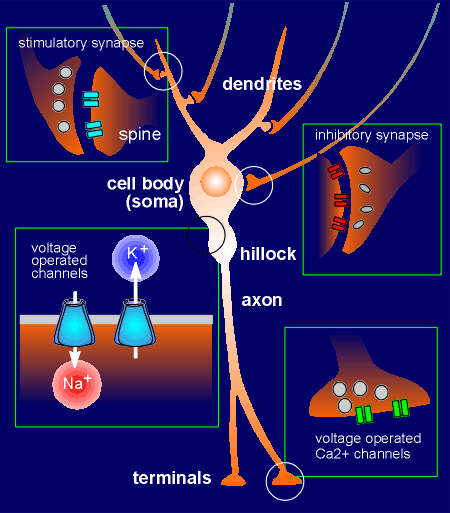
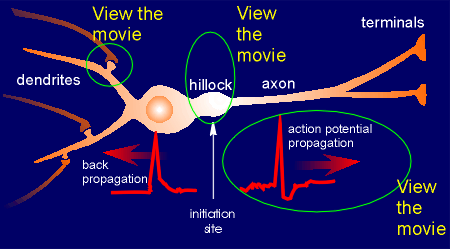
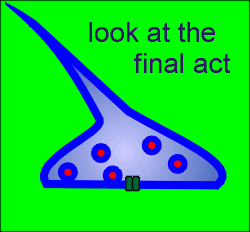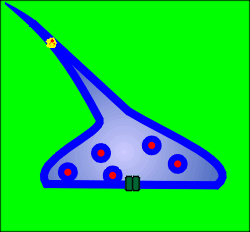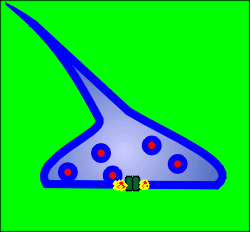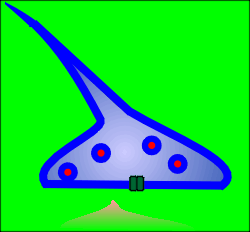
Given to the right is a representation of a typical neuron with dendrites, a cell body, a hillock, an axon and terminals.
Stimulatory synapses (which generate excitatory postsynaptic potentials, EPSPs) are generally found among the dendrites.
These are often on spines that extend from the dendrite.
Inhibitory synapses (which generate inhibitory postsynaptic potentials, IPSPs) are generally found on the cell body of the neuron.
Recent studies have shown that within the postsynaptic neuron there are special anchor proteins to hold receptors in the synapse.
 The link to the left will give you more details on anchoring proteins.
The link to the left will give you more details on anchoring proteins.
The secretory vesicles in the presynaptic inhibitory neuron have often an oval shape whereas in presynaptic stimulatory neurons they are generally round.
Voltage-operated ion channels are found in the hillock.
 These are activated by membrane depolarization to generate an action
potential which is progagated along the axon through the action of still more
voltage-operated ion channels.
These are activated by membrane depolarization to generate an action
potential which is progagated along the axon through the action of still more
voltage-operated ion channels.
When action potentials reache the terminals they cause opening of voltage-operated Ca2+ channels.
The influx of Ca2+ is the signal for exocytosis of secretory vesicles and thus the neuron releases neurotransmitter.
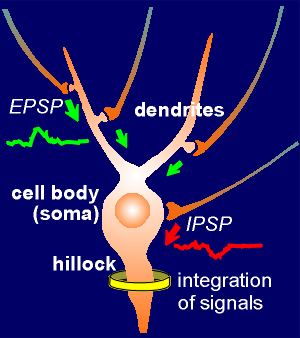 The hillock integrates signals
The hillock integrates signalsThe EPSPs are generated in the spines by the action of ligand-operated ion channels.
They propagate to the hillock where they are integrated.
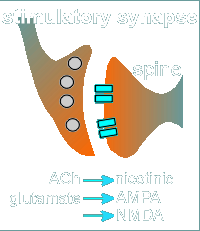 For example acetylcholine (ACh) working through a nicotinic receptor or
glutamate working through a AMPA or NMDA receptor can generate EPSPs.
For example acetylcholine (ACh) working through a nicotinic receptor or
glutamate working through a AMPA or NMDA receptor can generate EPSPs.
The size of the EPSP depends primarily on (1) the strength of the presynaptic signal (i.e. how much neurotransmitter was released) and (2) the sensitivity of the postsynaptic membrane to the neurotransmitter.
The postsynaptic sensitivity is most often determined by the number of receptors (the higher the receptor density the higher the sensitivity to the transmitter).
The EPSPs spread out from the site where they were generated by passive diffusion and, as they spread out, they dissipate (get smaller).
The single most important aspect concerning signal integration is how big the EPSP is when it reaches the site of signal integration in the hillock.
An EPSP generated at a synapse close to the hillock will experience less dissipation than an EPSP generated at a synapse far from the hillock.
Thus an important consideration for the working of a neuron is where on the dendritic field does one find the synapses.
Synapses close to the cell body (and thus close to the hillock) tend to be more important than synapses at more distal sites in the dendritic field.
It is now known that in some neurons the synaptic potential in the dendrite can activate dendritic voltage-gated ion channels to generate local dendrite spikes which propagate to the cell body.
In such neurons synapse in the outermost reaches of the dendritic field can make an important contribution to events in the cell body.
__________________
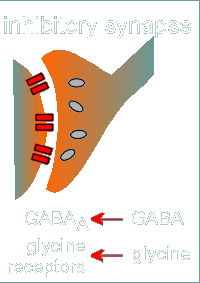 As already mentioned, the inhibitory synapses are generally located on the
cell body.
As already mentioned, the inhibitory synapses are generally located on the
cell body.
These can be GABAergic, with GABA as the neurotransmitter working through the GABAa receptor.
Another important inhibitory neurotransmitter is glycine, which works through a family of glycine receptors closely related to GABAa receptors.
An EPSP propagating through the cell body can be attenuated (made smaller) before it reaches the hillock if it encounters an IPSP generated at a inhibitory synapse.
As with the EPSP, the strength of the IPSP is determined by the strength of the presynaptic signal (how much neurotransmitter is released) and sensitivity of the postsynaptic membrane (how many receptors are present to receive the signal).
The important question for signal integration of the EPSPs and IPSPs is the summation in the hillock .... does the summation give a depolarization and, if so, how large a depolarization.
If the depolarization is sufficiently large to activate voltage-operated Na+ channels in the hillock then an action potential is generated.
 The potential at which the voltage-operated Na+ channels fire is known as
threshold.
The potential at which the voltage-operated Na+ channels fire is known as
threshold.
In the animation to the right note that only the EPSP that reached threshold generated an action potential.
Also note that the voltage-operated channel that opens at threshold is the Na+ channel.
At the top of the action potential this Na+ channel closes and, at this high potential, the K+ voltage-operated channel opens.
With the efflux of K+ the membrane potential quickly returns to the resting potential of ~70 mV.
The Na+/K+ ion balance is quickly restored by the action of the Na+-K+ ATPase.
 In the illustration to the left
are three links to animations prepared for the Book "Neurobiology"
by Gary Matthews.
In the illustration to the left
are three links to animations prepared for the Book "Neurobiology"
by Gary Matthews.
The first illustrates events in the synapse and the second gives another view of the initiation of an action potential at the hillock.
The third shows how the action potential is propagated along the axon, in the direction of the terminals, by opening Na+ and K+ voltage-operated channels that are distributed along the axon.
This latter movie also includes propagation along myelinated axons.
__________________
Recent research has shown that the action potential created at the hillock travels not only down the axon but in some neurons can also travel in the opposite direction through the cell body to the dendrites.
This is called "back propagation" of the action potential.
This means that the dendrites (and the synapses) receive information from the hillock concerning signal integration (i.e. did the neuron fire off an action potential after integrating all the EPSPs that were sent down from the synapses in the dendritic field).
Back propagation of the action potential is an important in the cascade of events that concern learning and memory (to be discussed in the lectures).
 When the Na+ driven action potential reaches the terminals it causes
opening of voltage-operated Ca2+ channels.
When the Na+ driven action potential reaches the terminals it causes
opening of voltage-operated Ca2+ channels.
A Ca2+ microdomain forms around each Ca2+ channel.
If a docked and "primed" secretory vesicle (i.e. a secretory vesicle that is ready for exocytosis) is found within the elevated Ca2+ then exocytosis occurs and neurotransmitter is released into the extracellular space of the synapse.
With closure of the ion channel the elevated intracellular Ca2+ quickly dissipates, the Ca2+ being bound by the Ca2+ buffering proteins or is pumped out of the cell or into intracellular compartments.
The released neurotransmitter acts on post-synaptic ion channel receptors to generate new signals and to participate in new integrative events in the flow of information through the central nervous system.
 In conclusion, ion channels and ion
channel receptors are of critical importance to the function of neurons in receiving and
processing information and in the generation of neuronal output.
In conclusion, ion channels and ion
channel receptors are of critical importance to the function of neurons in receiving and
processing information and in the generation of neuronal output.
 This is the end of the module "The Neuron"
This is the end of the module "The Neuron"