 PP-1 and PP-2A have a
particularly broad specificity and can remove phosphates that were transferred by PKA,
PKC, Ca2+/calmodulin protein kinase II and a number of other kinases that have not been
discussed in this tutorial.
PP-1 and PP-2A have a
particularly broad specificity and can remove phosphates that were transferred by PKA,
PKC, Ca2+/calmodulin protein kinase II and a number of other kinases that have not been
discussed in this tutorial.Protein phosphatases catalyze the hydrolysis of the ester bond of phosphorylated amino acids.
This releases inorganic phosphate and the unphosphorylated protein.
A limited number of multifunctional serine/threonine phosphatases account for most of the phosphatase activity in a cell.
They are categorized into six groups (1, 2A, 2B, 2C, 4, and 5) on the basis of, among others, their substrate specificity (type 3 has apparently disappeared in the mist of time!).
Only protein phosphatase 2B (PP-2B, also called calcineurin) directly responds to second messengers, namely it is activated by Ca2+/calmodulin and thus directly responds to increase in intracellular Ca2+.
 PP-1 and PP-2A have a
particularly broad specificity and can remove phosphates that were transferred by PKA,
PKC, Ca2+/calmodulin protein kinase II and a number of other kinases that have not been
discussed in this tutorial.
PP-1 and PP-2A have a
particularly broad specificity and can remove phosphates that were transferred by PKA,
PKC, Ca2+/calmodulin protein kinase II and a number of other kinases that have not been
discussed in this tutorial.
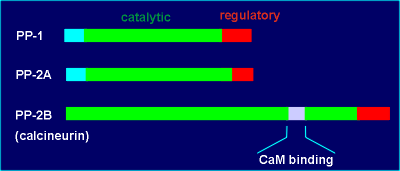
The catalytic domains of PP-1, PP-2A and PP-2B are highly homologous.
Also, each of these phosphatases possesses a regulatory domain in the C-terminal.
These regulatory domains are sites for kinase-induced phosphorylations.
The phosphatases are often anchored to specific sites in the cell (this will be considered further in the module "supramolecular signaling complexes" of this tutorial), and phosphorylations can affect anchoring and thus phosphatase function.
For example, phosphorylation of PP-1 regulatory domain in the liver releases the phospatase from its anchoring protein.
Consequently the dephosphorylation of substrate is reduced because of the reduction in the local concentration of PP-1.
Also, the released PP-1 can now interact with inhibitory proteins in the cytosol, the inhibitory activity of which is regulated by kinases.
From the above it is obvious that the activity of protein phosphatases is carefully regulated and thus play a crucial role in cell signaling.
The substrate specificity of calcineurin (PP-2B) and Ca2+ calmodulin protein kinase II (CaM kinase II) have little overlap.
Thus a rise in Ca2+ does not lead to a simple cycle of phosphorylation/dephosphorylation by these calmodulin-dependent enzymes.
Also, the Ca2+-calmodulin sensitivities of the two enzymes is quite different, with weak, low frequency stimuli selectively activating calcineurin whereas strong high frequency stimuli activate both calcineurin and CaM kinase II.
These differences may give these proteins an important function in neurons where low frequency discharges are associated with synaptic weakening (presumably brought about by calcineurin regulated pathways) and high frequency discharges cause synaptic strengthening (presumably through CaM kinase II regulated pathways).
As with PP-1, cytosolic factors are also involved in the regulation of calcineurin activity.