 They are called G proteins because
they are GTPases (they bind GTP and hydrolyze it to GDP).
They are called G proteins because
they are GTPases (they bind GTP and hydrolyze it to GDP).The principle of receptor signaling through G proteins is that the ligand induces a change in the sturcture of the receptor such that the receptor-ligand complex can activate the G protein (see movie to the right).
Intracellular loops of the receptors are responsible for the activation of the G proteins.
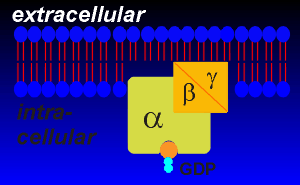
The G proteins associated with receptor transduction are the so-called "trimeric" G proteins because they are composed of three subunits, alpha, beta and gamma.
The trimeric designation distinguishes the receptor-associated G proteins from smaller intracellular "monomeric" G proteins that are involved in vesicular traffic and other processes within the cell.
 They are called G proteins because
they are GTPases (they bind GTP and hydrolyze it to GDP).
They are called G proteins because
they are GTPases (they bind GTP and hydrolyze it to GDP).
They are found embedded within the inner envelope of the lipid bilayer.
Alpha subunits have molecular weights of ~45,000 daltons and beta and gamma subunits are around ~20 - 30,000 daltons each.
The binding of GTP and its conversion to GDP is critical to the function of the G proteins.
The binding of GTP activates the G protein for interaction with effector proteins.
The activation step is illustrated in the animation to the right.
With the exchange of GDP for GTP the alpha and the beta/gamma subunits fall apart, each now in an active form to activate (or inactivate) effector proteins.
The beta and gamma subunits are very tightly bound and generally do not come apart.
The exchange of GDP for GTP is the most difficult step in the GTPase cycle of this protein (that's why the GTP is having such difficulty with the exchange in the animation).
This exchange is the rate-limiting step which determines how fast the G protein hydrolyzes GTP to GDP.
 Illustrated to the left is the inactivation of the G protein.
Illustrated to the left is the inactivation of the G protein.
The hydrolysis of GTP to GDP returns the G protein to the inactive state.
Here the inherent GTPase activity of the alpha subunit hydrolyzes GTP to GDP.
In the GDP-bound form the alpha subunit can now bind with the beta/gamma subunit and a trimeric inactive complex is formed.
As outlined above, the G protein catalyzes the hydrolysis of GTP, with the rate limiting step in the GTPase cycle being the exchange of GDP for GTP.
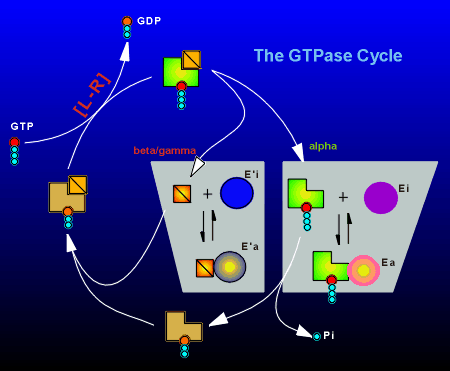 The
ligand-receptor complex (indicated as [L-R] in the figure to right) facilitates this
exchange process.
The
ligand-receptor complex (indicated as [L-R] in the figure to right) facilitates this
exchange process.
In the active GTP-bound form the alpha subunit has two choices:
......[1] it can hydrolyze GTP to GDP (and thus return to the inactive state), or,
......[2] it can combine with an inactive effector protein (Ei) and activate it (Ea).
Likewise the free beta/gamma subunit can interact with an effector (E') to take it from the inactive to the active state (E'i to E'a).
At rest, the bulk of the G proteins are in the inactive GDP-bound trimeric form.
To the left are two animations, one showing the basal GTPase activity, in the absence of an active ligand-receptor complex, and the other showing the GTPase activity in the presence of an active ligand-receptor complex.
Under basal conditions (i.e. with no active ligand-receptor complex present) there is low GTPase activity so that only once in a while is a GDP exchanged for a GTP with subsequent hydrolysis of GTP.
Thus the alpha subunit is only very infrequently in the active GTP bound form where it can activate effector proteins.
Likewise, the beta/gamma subunit finds itself only very infrequently in the free dimer form under basal conditions.
In the presence of an active ligand-receptor complex the exchange of GDP for GTP is facilitated, thus the ligand-receptor complex is said to act as an enzyme.
Now the G protein find itself more frequently in the active form (i.e. free alpha and free beta/gamma dimers), where it can activate effectors.
One [L-R] complex may catalyze the activation of many hundreds of G proteins before it itself is inactivated.
In this sense it acts as a true enzyme and gives a tremendous amplification to the action of ligands (a small amount of ligand activates a few receptors but each [L-R] complex activates many hundreds of G proteins).
This is one of the powerful features of receptor signaling through G proteins.
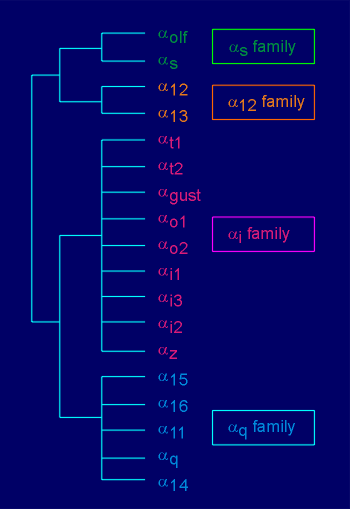 There are families of G
protein alpha subunits
There are families of G
protein alpha subunitsG proteins take their name from the alpha subunit (i.e. Gs possesses the alpha(s) subunit, Gi the alpha(i) subunit, etc).
There is a specificity in the substrate preference of alpha subunits.
For example alpha(s) stimulates the effector enzyme adenylyl cyclase: the "s" stands for "stimulation".
Likewise, the alpha(i) inhibits adenylyl cyclase: the "i" stands for "inhibition".
In general alpha(q) stimulates the enzyme phospholipase C beta (the origins of the designation q is unclear to me; in earlier literature this subunit was designated "p", presumably the "p" for phospholipase C beta).
There are at least 18 different alpha subunits (listed to figure to the right).
On the basis of amino acid homology these can be classified into 4 families, the alpha(s),alpha(12), alpha(i) and alpha(q) family.
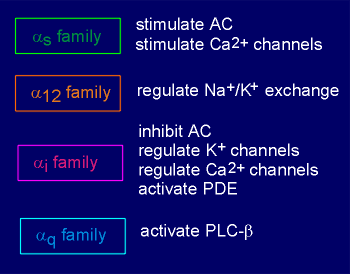 Alpha subunits can have more than one effector
(list to right)
Alpha subunits can have more than one effector
(list to right)
For example, alpha(i), besides inhibiting adenylyl cyclase, has been reported to interact with and stimulate K+ channels on the membrane (thus causing hyperpolarization and inhibition of the cell).
This same alpha subunit has also been reported capable of inhibiting voltage-operated Ca2+ channels on the membrane.
Thus this subunit has general inhibitory effect on the cell, whether it works through adenylyl cyclase, K+ channels, or Ca2+ channels.
The closely related family member alpha(o) is also reported to act on K+ channels and probably Ca2+ channels as well.
Some of the G proteins are involved in sensory transduction (transduction of sensory information such as light, smell or taste into receptor cell potentials).
 For
example, alpha(t), which is in the G(i) family, activates a phosphodiesterase (an enzyme
for the breakdown of cyclic nucleotides) in the rod cells of the eye.
For
example, alpha(t), which is in the G(i) family, activates a phosphodiesterase (an enzyme
for the breakdown of cyclic nucleotides) in the rod cells of the eye.
Alpha(olf) is found in the olfactory system where it participates in signal transduction of odorants and alpha(gust) is found in the gustatory system where it is involved in signal transduction of taste.
Use the link to the right to learn more about the role of G proteins in sensory transduction.
 The toxins
produced by the bacteria causing cholera and whooping cough (cholera toxin and pertussis
toxin, respectively) have been found to work by acting on G proteins.
The toxins
produced by the bacteria causing cholera and whooping cough (cholera toxin and pertussis
toxin, respectively) have been found to work by acting on G proteins.
An analysis of their action on G proteins has led to a better understanding of how these bacteria can disrupt normal body function.
The toxins have also become a valuable research tool in determining if hormones and neurotransmitters work through G protein mechanisms.
The link to the left gives an overview of the working of these two toxins and also an illustration of how the toxins can be used in research to probe cellular function.
There are at least 5 beta subunits and 12 gamma subunits.
In general, beta/gamma dimers have much less target specicity than alpha subunits (i.e. they interact with a large spectrum of effectors).
The nonspecific beta/gamma effects are perplexing since activation of all G proteins gives rise to free beta/gamma dimers.
It is perplexing because it is unclear what the point is of having specific alpha targets, if the beta/gammas are acitvating everything!.
One possible explanation is that beta/gamma in general must be present in far higher concentrations than alpha in order to exert their effects.
Thus, beta/gamma may provide a general readout for the cell of the total incoming information transmitted by G proteins.
Alternatively, beta/gamma dimers may be generated in signaling domains on the membrane which possess only certain effectors (and thus inappropriate activations are impossible).
Signaling domains will be discussed in greater detail in the module "Supramolecular Signaling Complexes, later in this tutorial.
 Finally, there is one
beta/gamma target which warrents special attention, namely beta-adrenergic receptor kinase
(beta-ARK), renamed G protein-coupled receptor kinase (GRK).
Finally, there is one
beta/gamma target which warrents special attention, namely beta-adrenergic receptor kinase
(beta-ARK), renamed G protein-coupled receptor kinase (GRK).
This effector provides an internal negative feedback loop to the working of G protein receptors in general.
Use the link to the left to learn more about this specific beta/gamma effector.