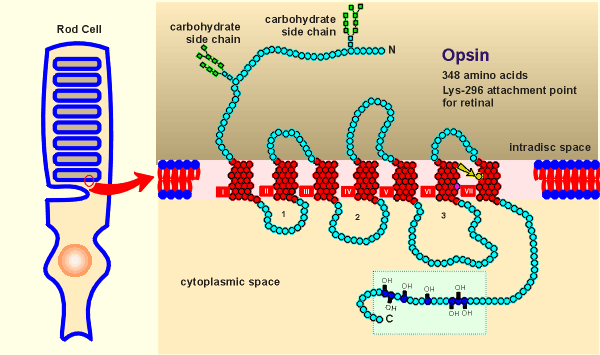
 To the right is the
structure of opsin, which is found embedded in the membrane of intracellular discs of the
rod cells.
To the right is the
structure of opsin, which is found embedded in the membrane of intracellular discs of the
rod cells.The first sensory transduction system where a link was made between sensory transduction and G proteins concerned the eye.
Within the rod cells of the retina of the eye the light-sensitive protein rhodopsin absorbs light leading to chain of events ultimately seen as light perception.
Rhodopsin consists of two parts, the light sensitive molecule retinal and the protein opsin.
 To the right is the
structure of opsin, which is found embedded in the membrane of intracellular discs of the
rod cells.
To the right is the
structure of opsin, which is found embedded in the membrane of intracellular discs of the
rod cells.
The structure looks almost the same as the beta-adrenergic receptor!
In fact the structure of opsin was know long before any of the structures of G protein-coupled receptors were known (rods have very high levels of rhodopsin and thus biochemists were able to purify the opsin protein and sequence it).
The real surprise came when the first G protein receptor structure was elucidated and found to have sequence homology with opsin.
Like the beta-adrenergic receptor, opsin has seven transmembrane domains, it is glycosylated at the N-terminal and it has a number of serine/threonines at the C-terminal which are sites for phosphorylations by kinases (a regulatory domain).
 Like receptors, opsin
binds a ligand, in this case not a neurotransmitter but the light-sensitive molecule
retinal (the binding site is Lysine-296 in the 7th transmembrane region, indicated by the
yellow arrow in figure above).
Like receptors, opsin
binds a ligand, in this case not a neurotransmitter but the light-sensitive molecule
retinal (the binding site is Lysine-296 in the 7th transmembrane region, indicated by the
yellow arrow in figure above).
Like the neurotransmitter, the retinal nestles deep into the pocket formed by the seven transmembrane regions.
A difference with neurotransmitters is that, once nestled in the opsin, the retinal is then covalently bound to the opsin (bond at lysine-296), thus forming rhodopsin.
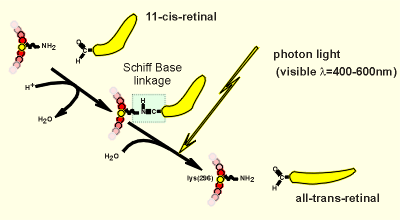 Shown to the right is the
linkage of retinal with opsin.... the aldehyde group of retinal can form a so-called
"Schiff base linkage" with the amino group on lysine-296.
Shown to the right is the
linkage of retinal with opsin.... the aldehyde group of retinal can form a so-called
"Schiff base linkage" with the amino group on lysine-296.
A photon of light in the visible spectrum can break this linkage.
The release of the retinal causes a transformation change in the opsin (comparable to the transformation change in a receptor caused by ligand binding).
The next step in this sensory transduction is that the opsin, in its transformed conformation, can activate a G protein.
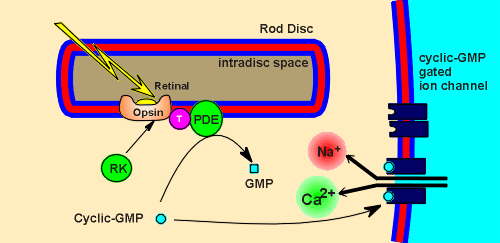 The G protein
is called transducin (T in figure to left).
The G protein
is called transducin (T in figure to left).
The alpha subunit of transducin (alpha(T)) activates an enzyme called phosphodiesterase (PDE).
PDE is responsible for the breakdown of a cyclic nucleotide, cyclic-GMP, to GMP.
Cyclic-GMP activates an ion channel on the membrane of the rod cell.
The cyclic-GMP gated ion channel is a cation channel that allows entrance of both Ca2+ and Na+.
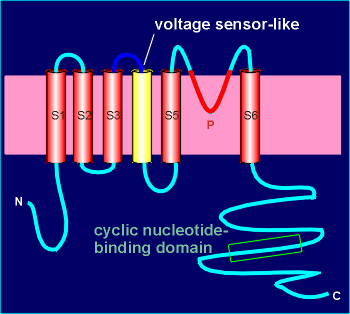 The channel is
composed of 4 subunits with high homology to voltage-operated channels (review structure of voltage-operated channels?).
The channel is
composed of 4 subunits with high homology to voltage-operated channels (review structure of voltage-operated channels?).
It even has a structure in the 4th transmembrane region which resembles a voltage-sensor, although it is not voltage sensitive.
Like voltage-operated channels, the ion channel is constructed from P domains of the subunits.
Each subunit has a cyclic-GMP binding domain in the C terminal region (thus intracellular).
Under conditions of high cyclic-GMP (i.e. in the dark) there is an influx of Na+ and Ca2+ and thus a depolarization of the rod cell.
In the light there is a G protein-induced activation of PDE, thus a breakdown cyclic-GMP, and the channels close to give a hyperpolarization of the rod cell.
So, strange as it seems, hyperpolarization in light, depolarization in dark.
In the cytosol of the rod cell is a kinase, rhodopsin kinase (RK), which can phosphorylate opsin and, in doing so, desensitize the rhodopsin to light.
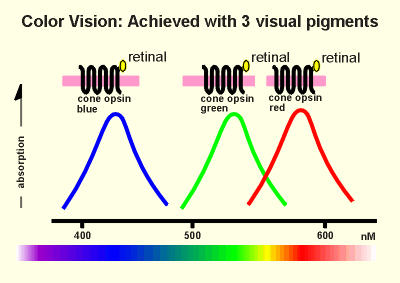 Color transduction in
cone cells of the retina works almost the same as described above for the rod cells.
Color transduction in
cone cells of the retina works almost the same as described above for the rod cells.
The three opsins involved in color vision have slightly different amino acid sequences and thus slightly different properties.
When combined with retinal one of these opsins absorbs in the blue, one in the green and the other in the red? (rod opsin, in contrast, has a very broad absorption spectrum in the visible light range).
Thus we have cone opsin blue, cone opsin green and cone opsin red in the three different types of cone cells responsible for color vision.
Each of the opsins uses retinal to absorb the light.
The protein moiety determines which wave lengths of light are absorbed.
It is clear that the mechanism of sensory transduction in the rod cells has a lot in common with signal transduction of neurotransmitters:
(1) The opsin has high homology with the G protein-coupled receptors,
(2) The retinal nestles in the opsin much like a neurotransmitter in a receptor,
(3) The transducin has high homology with the G proteins which regulate neurotransmitter receptors.
(4) The transducin activates an effector enzyme.
There was two apparently unique features to sensory transduction in the rods.
First, the effector is a phosphodiesterase (and not any of the other known enzyme effectors activated by neurotransmitters such as adenylyl cyclase or phospholipase C beta).
Second, the cyclic nucleotide (cyclic GMP) is acting directly on an ion channel rather than activating a protein kinase.
The first feature remains unique to light transduction (as far as I know receptor-regulated phosphodiesterases occurs only in sensory transduction).
The second feature, cyclic nucleotide gated ion channels, has now been found elsewhere.
First, such channels were found to function in the olfactory system where they were involved in olfactory sensory transduction.
Again, the system is working exactly like neurotransmitters and their G protein-coupled receptors.
The odorants represent the ligands for the receptor proteins (which have high homology with neurotransmitter receptors).
Receptor activation leads to activation of a G protein, in this case Golf, the alpha subunit of which is in the alpha(s) family.
Alpha(olf) activates adenylyl cyclase for the production of cyclic AMP.
The cyclic AMP then directly activates a cyclic nucleotide gated Na+ channel, in analogy with events in rod cells, leading to olfactory receptor cell depolarization.
From the human genome project it has been estimated that there are 700 - 800 different odorant receptors in the olfactory system.
In the gustatory system (taste) a similar though less well understood system is working where gustatory receptors activate G(gust) to generate activate alpha(gust).