 It has been found that the toxic action of two
different bacterial toxins, cholera toxin and pertussis toxin, are through their
actions on G proteins.
It has been found that the toxic action of two
different bacterial toxins, cholera toxin and pertussis toxin, are through their
actions on G proteins.  It has been found that the toxic action of two
different bacterial toxins, cholera toxin and pertussis toxin, are through their
actions on G proteins.
It has been found that the toxic action of two
different bacterial toxins, cholera toxin and pertussis toxin, are through their
actions on G proteins.
Both toxins act as enzymes modifying the alpha subunit of G proteins.
The modification concerns the addition of a ADP-ribose to the alpha subunits of G proteins.
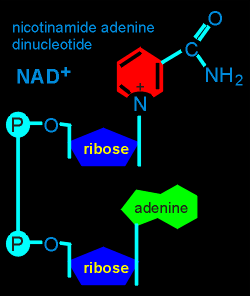
ADP-ribose is found within the structure of NAD, an important electron acceptor in many biochemical pathways within cells.
In a so-called "ADP-ribosylation" of a protein the ADP-ribose is transferred from NAD to the protein.
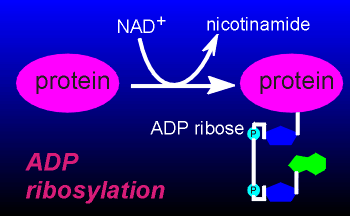 It is this reaction
which the bacterial toxins catalyze and, in doing so, drastically alter the function of G
proteins.
It is this reaction
which the bacterial toxins catalyze and, in doing so, drastically alter the function of G
proteins.
As outlined below the effects of cholera toxin and pertussis
toxin, while both depending on ADP-ribosylation, are quite different.
It took about 100 years from the time of the discovery of the cholera bacterium, vibrio cholerae (in 1884) to expose the real cause of the disease cholera, namely the effect of the toxin on G proteins.
The bacterium produces the toxin, a large protein, one part of which can penetrate the cell membranes where it ADP-ribosylates the alpha subunit of Gs.
The alpha(s) subunit can no longer hydrolyze GTP and thus turn itself off.
Consequently it remains active, disrupting the function of epithelial cells in the intestine resulting in an enormous loss of water.
Cholera toxin is used in research to determine if a receptor mechanism is working through Gs.
If the ligand-receptor mechanism is working through Gs, then cholera toxin should potentate the action.
This is because the ligand-receptor accelerates the production of active alpha(s) which then becomes blocked in the active form through the action of the toxin.
Thus there is a build-up of activated alpha(s).
If the mechanism is not working through Gs then cholera
toxin has no effect on the action of the ligand-receptor complex.
Pertussis toxin is produced by the bacterium Bordetella pertussis.
When this bacterium infects the lungs it causes inflammation and mucus production.
This is accompanied by very loud coughing, thus the name "whooping cough" to describe the illness associated with this bacterium.
Pertussis toxin works very similar to cholera toxin with two important differences, namely the target for the ADP-ribosylation is Gi proteins and the ribosylation locks the G protein in the inactive form.
 Another interesting difference between the two toxins is that the catalytic
subunit of pertussis toxin somehow manages to completely penetrate the membrane and come
into the intracellular compartment.
Another interesting difference between the two toxins is that the catalytic
subunit of pertussis toxin somehow manages to completely penetrate the membrane and come
into the intracellular compartment.
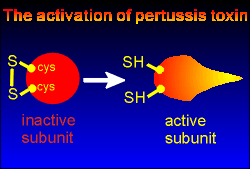
In the cytosol the toxin subunit must first be activated in order to ribosylate the G proteins.
This activation involves a reduction of a disulfide bond between cysteine residues within the toxin, a reduction that is carried out by an enzyme present in the cell.
With reduction of the disulfide bond the toxin comes into its active form, ready to ADP-ribosylate Gi subunits.
Because pertussis toxin acts specifically on Gi proteins it can be used as a tool to identify receptor ligands which work through this G protein.
 Given to the right is an experiment illustrating this principle.
Given to the right is an experiment illustrating this principle.
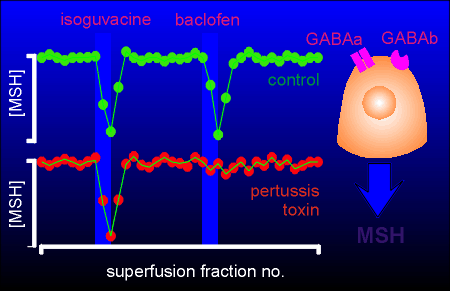
The experiment involves the regulation of release of melanophore stimulating hormone (MSH) from melanotrope cells of the pituitary gland.
The neurotransmitter GABA inhibits MSH release from this cell through action on both GABAa and GABAb receptors (the former is a chloride ion channel, the latter works through G proteins).
In the experiment to the right pituitary tissue was put in a superfusion chamber and superfusion medium pumped over the tissue and collected in a fraction collector.
The amount of MSH in each fraction was determined with a radioimmunoassay.
In the control tissue a pulse of both isoguvacine (a GABAa receptor agonist) and baclofen (a GABAb receptor agonist) inhibited MSH secretion.
In pertussis toxin-treated tissue (treated overnight with the toxin) isoguvacine was still effective in inhibiting MSH secretion (as expected since it does not work through G proteins) but baclofen was no longer effective.
This data give strong evidence that baclofen (and thus the GABAb receptor) is working through the Gi protein in exerting its inhibitory action on this cell.