 The two most
common configuration in patch-clamp electrophysiology are the cell-attached and the
whole-cell configuration.
The two most
common configuration in patch-clamp electrophysiology are the cell-attached and the
whole-cell configuration. The two most
common configuration in patch-clamp electrophysiology are the cell-attached and the
whole-cell configuration.
The two most
common configuration in patch-clamp electrophysiology are the cell-attached and the
whole-cell configuration.
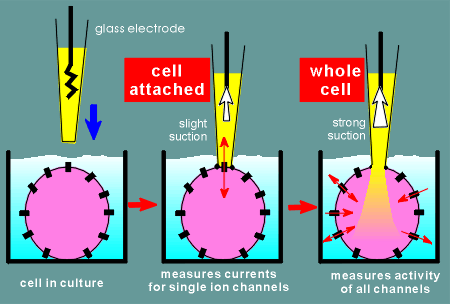
A glass electrode is gently brought onto the surface of a cell and a slight suction is applied to form a giga ohm seal on the membrane.
You are now in the cell attached configuration of the patch-clamp method.
With this configuration you can measure the electrical activity of ion channels (indicated as black objects on surface of cell) within the patch, usually a single channel.
With a strong suction one can go from the cell-attached configuration to the whole-cell configuration.
In the whole cell configuration one measures the electrical activity of all ion channels on the membrane of the cell.
If you are interested in recording one type of channel (e.g. perhaps only Na+ channels, or only Ca+ channels or only K+ channels) then specific channel blockers are introduced to the medium to block all other channel.
For example, a chemical called TTX is a general Na+ channel blocker and cobalt ions (Co2+) are general Ca2+ channel blockers.
Thus with TTX and Co2+ in the medium one can measure exclusively K+ cation channels.
In the whole-cell configuration the liquid in the electrode has ion concentrations that are the same as intracellular ion concentrations (i.e. high K+ and low Na2+).
When the patch breaks through to give the whole-cell configuration the liquid of the electrode mixes with the cytosol.
If the researcher wants to introduce molecules inside the cell (e.g. perhaps an antibody against a certain enzyme or an activator of a certain kinase) then he can simply put them in the liquid of the electrode.
When the patch breaks through the molecules will quickly mix with the cytosol.