24
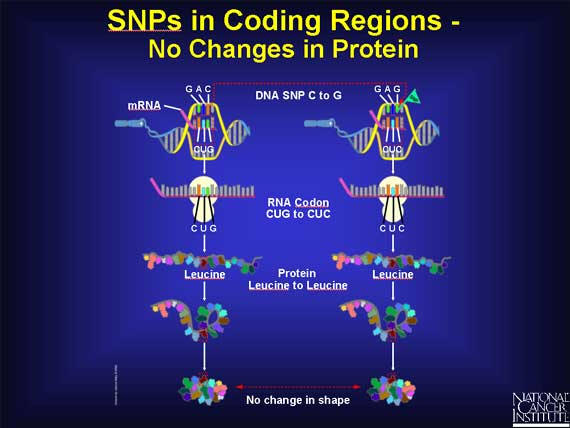
When a SNP occurs within a coding region, it is possible that there is no effect. For example, when the DNA sequence GAC becomes GAG, the codon in the mRNA changes from CUG to CUC. Since both codons stand for the same amino acid leucine, there is no change in the protein. The change in the DNA is called a silent change.
25
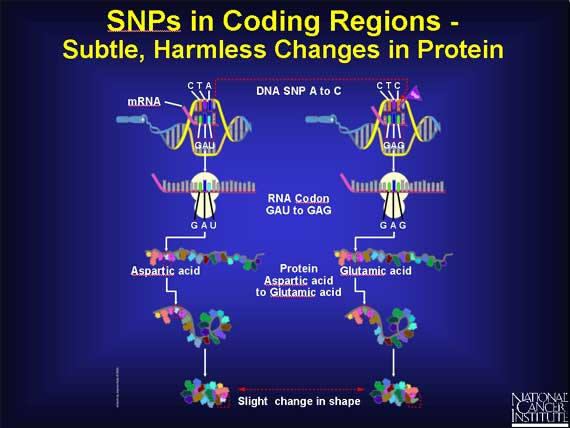
There are also SNPs within coding regions that lead to very subtle changes in proteins. For example, if a SNP causes the codon GAU to change to GAG, the amino acid changes from aspartic acid to glutamic acid. These amino acids have very similar chemical properties, but glutamic acid is just a little bigger. If the SNP causes a change in a part of the protein that is not important to its function, the result may be very subtle or totally harmless. The cell continues to function normally.
26
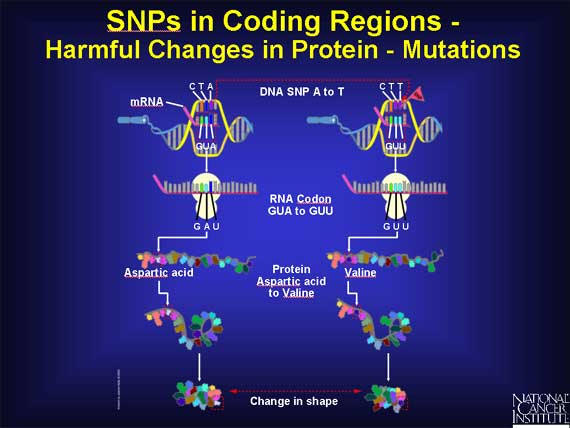
In another codon, the sequence GUA becomes GUU, and the amino acid called aspartic acid changes to valine in the protein. These two amino acids have very different chemical properties. The substitution of one for the other may, or may not, severely alter how the protein folds and functions, depending on where the change occurs in the protein. When the change in the protein does lead to disease symptoms in the patient, the SNP is harmful and is called a mutation.
An example of a SNP causing disease is found in sickle cell anemia. The change in one nucleotide base in the coding region for the hemoglobin beta gene causes the amino acid glutamic acid to be replaced by valine. As a result, the hemoglobin molecule can no longer carry oxygen as efficiently because the structure of the protein is changed dramatically.
27
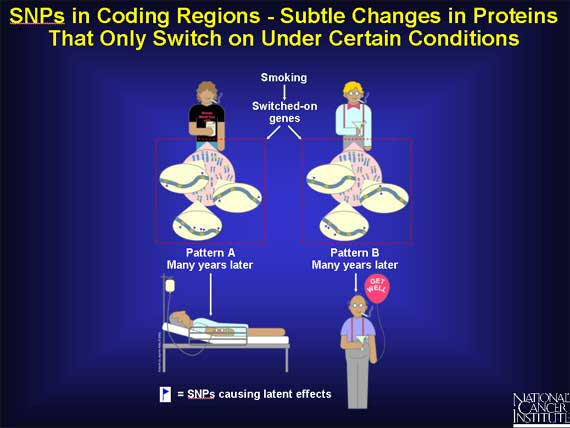
SNPs may cause subtle changes in a group of genes that under normal conditions are latent, i.e., they are switched "off". But when a person is exposed to precarcinogens or carcinogens, they can be switched "on". Since the proteins from these genes regulate how fast or how slowly the harmful agents are absorbed, bound, metabolized, and excreted from the body, even a small or subtle change in any one of them may alter a person's risk for cancer. It is important to remember that the SNP itself does no harm under normal circumstances. Only when a person is exposed to an environmental agent that is carcinogenic, does the SNP exert an influence.
28
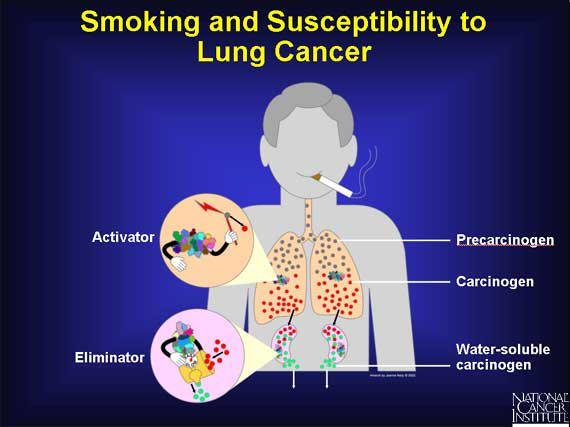
When a person smokes cigarettes, precarcinogens in the form of tobacco smoke enter a person's lungs and lodge in the fat-soluble area of the cells. They become bound to proteins that convert the precarcinogens into carcinogens. These reactive molecules are quickly handed over to detoxifying proteins that make the carcinogens water-soluble and allow the body to eliminate them into the urine, before they can damage the cell.
29
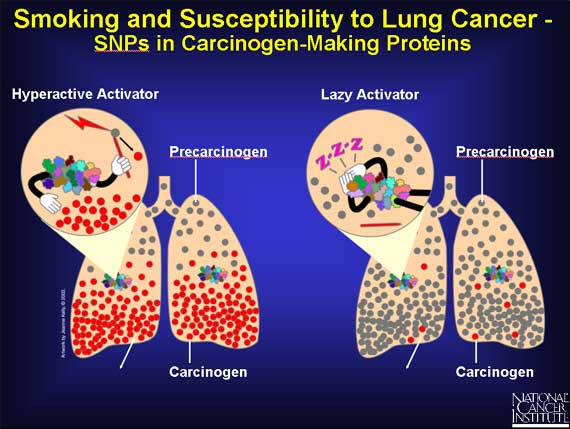
Because of SNPs, a person's genome may express a very active carcinogen-making protein, or a sluggish one, or something in between. A protein with a very active binding site can "grab" more precarcinogen than usual. Or the protein may convert the precarcinogen to the carcinogen at a faster rate. In both cases, more carcinogen molecules pile up in the lungs, causing damage to the cells' DNA, which can lead to cancer. On the other hand, if the SNPs result in a slow carcinogen-making protein, the lung is exposed to fewer DNA-damaging carcinogens, and the chance of cancer is reduced. But until the body is exposed to cigarette smoke, no effect is seen.
30
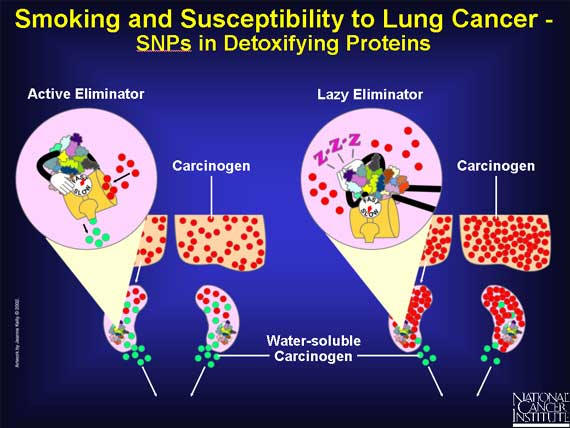
SNPs may also influence detoxifying enzymes that prepare carcinogens for elimination from the human body. SNPs that result in very active forms of detoxifying enzymes will remove the carcinogens quickly from the body, allowing less time for damage. SNPs that produce sluggish detoxifying enzymes will permit carcinogens to remain in the body for a longer time.
31
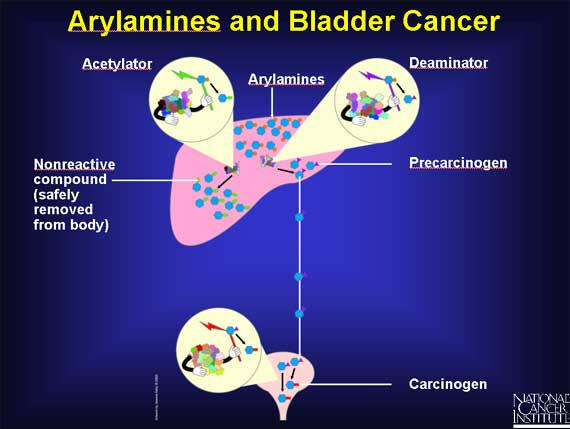
Some workers in the dye industry, after occupational exposure to arylamines, develop an increased risk of bladder cancer. Scientists suspect that SNPs may be involved. In the liver, arylamines can have two fates, because there are two enzymes available to act on them. An acetylator enzyme can deactivate arylamines, converting them into nonreactive compounds that are safely removed from the body. Or arylamines can be activated by a deaminator enzyme in the liver to become precarcinogens that are carried to the bladder. There they are converted into carcinogens.
32
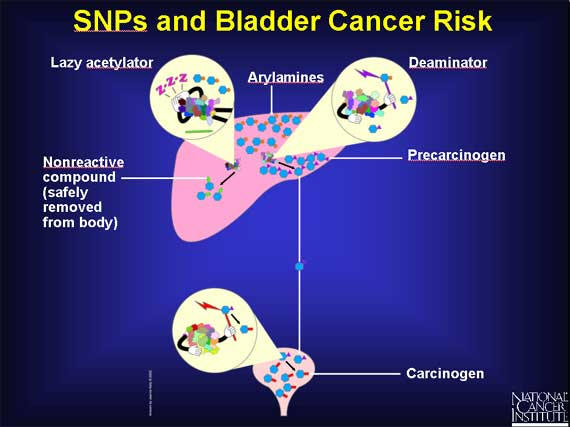
SNPs are responsible for the existence of several different slow forms of the acetylator enzyme. In individuals with a slow acetylator, the arylamines remain in the liver for a longer time, and more of them are converted to precarcinogens rather than nonreactive compounds. Thus, individuals with slow acetylators may be at increased risk for bladder cancer because they are exposed to more carcinogens in the bladder.
33
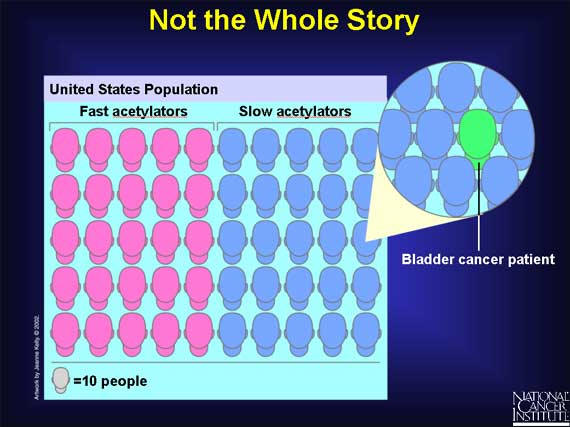
But SNPs are not always the entire story. Even among persons with slow acetylators who are exposed to arylamines, not everyone is at increased risk for bladder cancer. Research studies show that half the population in the United States have slow acetylators, and yet far fewer people, about one in 500, get bladder cancer. So having a "slow acetylator" does not automatically mean that you will get bladder cancer after arylamine exposure. Some other genes and their proteins must also be involved in affecting a person's risk for the disease. Researchers have yet to discover the full set of "risk" genes involved.