Neuronal outgrowth and axon guidance
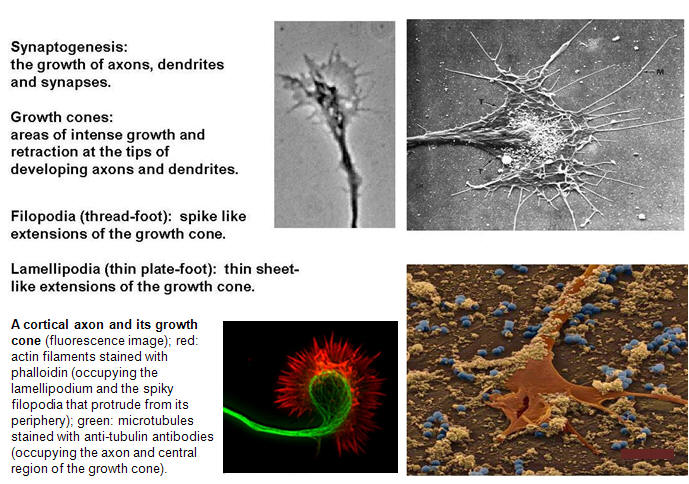
Perhaps the most remarkable feature of the nervous system is its highly ordered connections. We will here consider the processes that insure specific synaptic connections between neurons. Thus, one of the most intriguing problems in developmental neurobiology is that of how growing axons find the correct way to their proper target cells. Often axonal connections are organized in topographic maps, where neighboring cells of the projecting area are connected to neighboring cells in the target area, thus allowing a faithful transfer of positionally stored information from one area to another. A complex "wiring system" of nerve fibers connects the brain's more than 100 billion neurons. Studies on worms, flies, frogs and other animals are now identifying molecules that guide these fibers through the brain to their targets. These discoveries are revealing how the brain develops and may lead to ways of regenerating nerve fibers after injury or preventing wiring defects that result in disease. The brain's wiring system -- nerve fibers (axons) that grow along specific paths to connect with other fibers (dendrites) -- was first described more than a hundred years ago. Scientists learned that axons grow by following an elongated tip called a growth cone (Figure 1). Yet the systems that guide axons to connect with their targets are so complex that they are only now being unraveled. The correct formation of a network by neuronal cells appears to be established by the pathfinding of extending lamellipodia and filopodia at the growth cone. Filopodia, which initiate and elongate from the growth cone lamellipodia, are thin protrusions with actin bundles at their core (Figure 1). Their extension and retraction are initial events in the steering of a growth cone. Clarifying the molecular mechanisms by which filopodia extend and retract, and the signal pathways that control these events, is an important step in understanding how extracellular cues guide growth cones.
 Figure
1
Figure
1
A well-known example often used for neuronal outgrowth/axon guidance studies is the retinal ganglion cell (RGC), the only type of neuron that connects the eye to the brain. The eye is a peripheral outpost of the CNS where the RGCs reside. In the developing vertebrate visual system, retinal ganglion cells in the eye extend axons that navigate over a long distance to their synaptic targets in the midbrain. This impressive navigational feat underlies the precise wiring of the mature brain and is essential for building functional nerve connections. RGC axons navigate to their targets in a remarkably stereotyped and error-free manner and it is this process of directed growth that underlies the complex organization of the adult brain. The RGCs are the only retinal neurons to project into the brain and their peripheral location makes them an unusually accessible population of projection neurons for experiments involving in vivo gene transfer, anatomical tracing, transplantation and in vitro culture. These connections must thus be formed with extreme precision in order for an organism to obtain an accurate representation of the visual field. The retinotectal projection is the classical model system for studying topographic projections. RGC cell bodies and dendrites reside in the retina, while thier axons follow a stereotypic pathway through the diencephalon to innervate their target neurons in the optic tectum. We can manipulate and observe developing RGCs particularly well in tadpoles of the South African claw-toed frog Xenopus laevis or in zebrafish. With these developing visual systems one can study how neurotrophic molecules influence RGC axonogenesis, axonal vs. dendritic arborization, growth cone navigation, target recognition, and synaptogenesis in vivo (Figure 2).
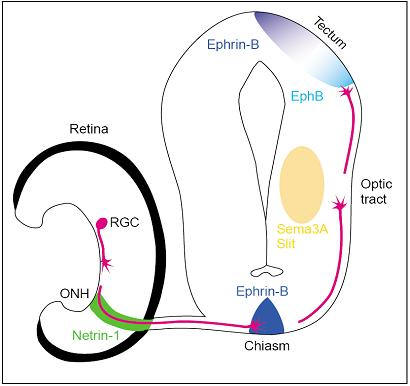
Figure 2. Diagram of the embryonic visual pathway. Guidance molecules belonging to the netrin, slit, semaphorin and ephrin families are expressed in multiple places along the pathway, in discrete segments, and serve to direct the growth of RGC growth cones. For simplicity, the positions of only a few cues are shown. ONH, optic nerve head; RGC, retinal ganglion cell.