 Shown here is the general structure of the receptor for
Platelet Derived Growth Factor (PDGF).
Shown here is the general structure of the receptor for
Platelet Derived Growth Factor (PDGF). Tyrosine kinase receptors have four structural or functional domains: (1) the extracellular ligand binding domain, (2) the transmembrane domain, (3) the intracellular catalytic domain and (4) the intracellular substrate or effector recognition domain for substrate binding.
 Shown here is the general structure of the receptor for
Platelet Derived Growth Factor (PDGF).
Shown here is the general structure of the receptor for
Platelet Derived Growth Factor (PDGF).
This receptor is considered a prototype for tyrosine kinase receptors.
The receptor has a large extracellular domain where ligand binding takes place.
It has a single transmembrane domain.
Intracellularly it has what is known as a split catalytic domain, with the ATP-binding site separate from the kinase site itself.
It also has a number of sites for substrate binding.
These sites contain the amino acid tyrosine which as you will see shortly, are important in substrate binding.
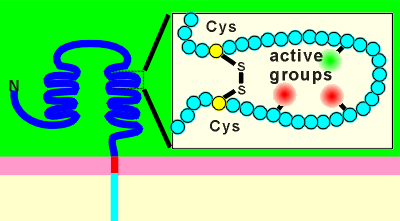 The large extracellular domain has many loops held together by disulphide
bridges.
The large extracellular domain has many loops held together by disulphide
bridges.
It is thought that one function of these bridges is to form a pocket for ligand binding.
The disulpide bridges would give structure to the ligand binding domain.
Active groups on side chains of amino acids within the loop may be held in the proper orientation for interaction with the ligand.
Active groups may be acetic groups (e.g.from the amino acids glutamic acid or aspartic acid) or basic groups (arginine or lysine).
Note: involvement of disulphide bridges in formation of ligand binding sites is also found in ion channel-type receptors and G protein-coupled receptors.
The tyrosine kinase receptors have only one transmembrane-spanning region. As outlined below this creates two potential problems for the functioning of this receptor type.
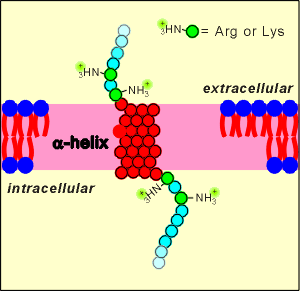 At one time it was questioned how such a large protein, with only one
transmembrane region, would be stable and stay in the membrane.
At one time it was questioned how such a large protein, with only one
transmembrane region, would be stable and stay in the membrane.
The expectation was that the receptor would be unstable and flip easily out of the membrane.
cDNA methods revealed the full sequence of these receptor proteins and it was discovered that they possess positively charged amino acids (lysine and arginine) near the membrane spanning region.
The idea is that these charged amino acids would give the receptors stability within the membrane.
The charged groups would not like the hydrophobic environment of the membrane, and thus it would be very difficult to pull the receptor out of the membrane.
In this way the receptor achieves stability within the membrane.
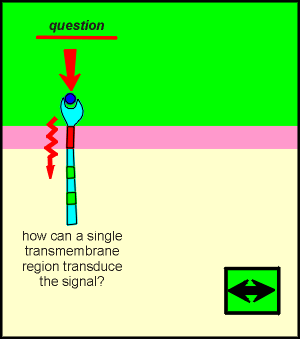
By comparison, G protein-coupled receptors, which do transduce information through the membrane regions, have 7 transmembrane domains to do the work.
The ligand induces transformation changes in the transmembrane domains.
These in turn interact to induce changes in intracellular domains.
For the tyrosine kinase receptors it has turned out that signal transduction does not involve the transmembrane region but rather that the ligands of TKRs induce the receptors to dimerize.
It is now known that, with ligand binding and dimerization, there is a rapid phosphorylation of tyrosines on the intracellular domains of the receptors.
This probably occurs via a transmolecular phosphorylation (each receptor phosporylates its partner).
Auto-phosphorylations within the ligand-receptor is the first step in the activation of a cell via TKRs.
Thus transduction of the signal through a transmembrane domain, to bring changes about inside the cell, is completely unnecessary for this receptor.
Rather, the ligand induces receptor dimers which bring the catalytic domains near substrate tryrosines on partner receptors.
In this way a change outside the cell (the presence of a message molecule) has brought about a change inside the cell (phosphorylation of intracellular domains of the receptor).
This is a form of transmembrane signaling.
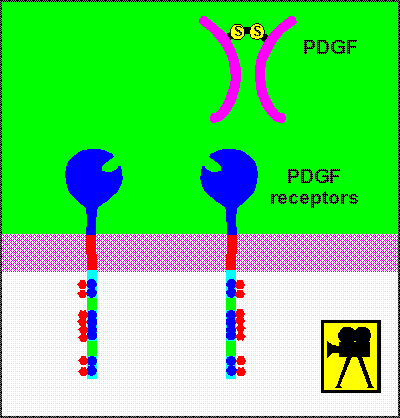
Dimer formation is a prerequisite for ligand activation of the receptor. Different ligands have different ways to achieve this goal. Outlined below are three examples of how different ligands activate their receptors.
Platelet derived growth factor (PDGF) is a dimer of two identical peptide chains held together by a disulphide bridge. Epidermal growth factor (EGF) induces a change in the extracellular domain so that receptors attract each other to form dimers. For insulin, the receptor is already a dimer waiting to be activated by the ligands.
No matter how they get together, the result is the same, a change outside has induced a change inside....transmembrane signaling!
This occurs on a number of tyrosine sites within the receptor (tyrosine, of course, because the receptor is a tyrosine kinase!).
It is believed that this phosphorylation is transmolecular (intermolecular), that is to say, each receptor phosphorylates its partner in the dimer complex.
It should now be clear....why dimers?
Dimers are necessary so that the catalytic domains come in contact with their target tyrosines on the partner receptor molecule.
The next step.......phosphorylation of substrate proteins inside the cell.
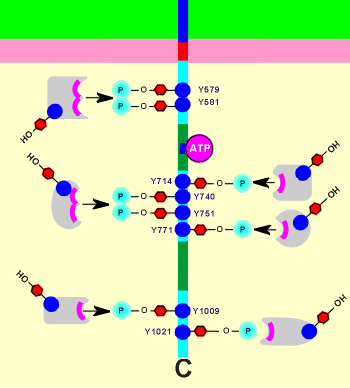 Intracellularly the TKR has a number of potential substrates.
Intracellularly the TKR has a number of potential substrates.
These substrates have recognition sites to make a binding with the receptor.
Shown to the right is a representation of the the intracellular domain of a PDGF receptor with its tyrosines phosphorylated (note: a monomere is shown but, as you have just seen, dimer formation would be necessary to achieve phosphorylation).
The phosphotyrosines of the receptor are important in substrate recognition; the position numbers of the phosphotyrosines within the protein structure are given in the figure; remember Y= tyrosine).
The substrates (shown in grey) bind to the phosphotyrosines.
This is the reason the transmolecular phosphorylations are so important.
If there is no phosphorylation of the receptor then there is no substrate recognition and the signaling fails.
Thus, the ligand-bound phosphorylated receptor dimer is the active complex.
The substrates themselves also possess tyrosines, to be phosphorylated by the receptor.
You will receive more information on the substrates in the next module of this tutorial, TKR Substrates.