 Steroid
hormones are produced primarily by the ovaries, testes and the adrenal cortex.
Steroid
hormones are produced primarily by the ovaries, testes and the adrenal cortex. Steroid
hormones are produced primarily by the ovaries, testes and the adrenal cortex.
Steroid
hormones are produced primarily by the ovaries, testes and the adrenal cortex.
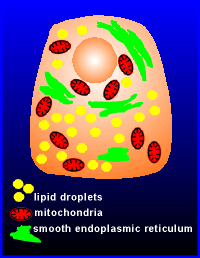
Steroid hormone producing cells have a very characteristic appearance, namely they are very rich in lipid droplets, mitochondria and smooth endoplasmic reticulum.
These three characteristics reflect the function of this cell to produce steroid hormones.
The lipid droplets are sites for the storage form of cholesterol, esterfied cholesterol (the cholesterol itself is produced in the liver and transported to other cells via the blood in a complex with proteins known as low-density lipoprotein particles, LDL).
Steroid hormones are not stored in the cell but rather synthesized on demand from cholesterol.
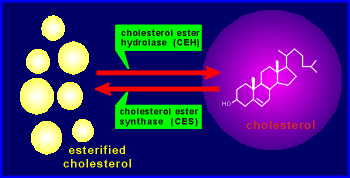 The esterfied
cholesterol is mobilized to form cholesterol, and ultimately to form steroid hormones,
by the enzyme cholesterol ester hydrolase, CEH.
The esterfied
cholesterol is mobilized to form cholesterol, and ultimately to form steroid hormones,
by the enzyme cholesterol ester hydrolase, CEH.
The enzyme cholesterol ester synthase (CES) is responsible for the opposite reaction.
Once the cholesterol is formed it enters the pathway for the production of steroid hormones.
Which steroid is produced depends on the cell type (and thus the profile of enzymes present in the cell).
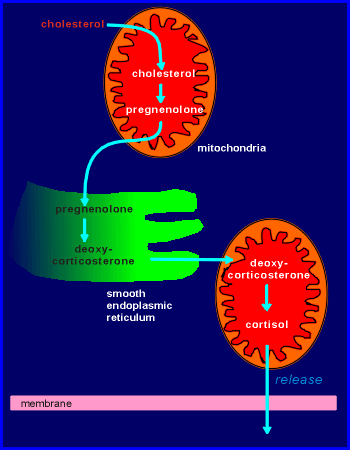 The synthesis of
steroid hormones is compartmentalized in the cell, some steps taking place in the
mitochondria and some in the lumen of the smooth endoplasmic reticulum (thus accounting
for the fact that the cell is rich in mitochondria and smooth ER).
The synthesis of
steroid hormones is compartmentalized in the cell, some steps taking place in the
mitochondria and some in the lumen of the smooth endoplasmic reticulum (thus accounting
for the fact that the cell is rich in mitochondria and smooth ER).
The pathway for the synthesis of cortisol, one of the major steroid hormones of the human adrenal, is shown in the illustration to the right.
Once formed, the cortisol has no problem in getting out of the cell because it is hydrophobic and can thus easily cross lipid bilayers.
Thus there is no secretory machinery (secretory vesicles, exocytosis machinery etc.) for the steroids, as there is for peptide hormone and neurotransmitters.
 The rate-limiting
step in the synthesis of the steroid hormones is the first step, the conversion of
esterfied cholesterol to cholesterol.
The rate-limiting
step in the synthesis of the steroid hormones is the first step, the conversion of
esterfied cholesterol to cholesterol.
As expected, this step is regulated in steroid hormone-producing cells.
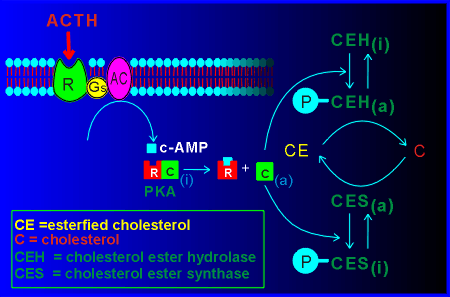
For the cells of the adrenal cortex producing cortisol, the regulation of this critical step is through the pituitary hormone adrenocorticotropic hormone (ACTH).
The ACTH receptor activates a Gs protein which in turn activates adenylyl cyclase (AC).
The cyclic AMP generated activates protein kinase A (PKA), the catalytic unit of which phosphorylates cholesterol ester hydrolase (CEH).
The activated CEH hydrolyzes the cholesterol esterase to form free cholesterol which is quickly utilized by the biosynthetic pathway producing cortisol.
The PKA also phosphorylates the enzyme cholesterol ester synthase.
This phosphorylation leads to an inactivation of the enzyme.
Thus the PKA stimulates production of cholesterol and inhibits production of cholesterol esters.
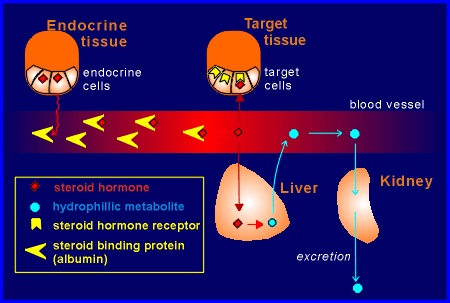 Most
steroid hormones have carrier proteins in the blood.
Most
steroid hormones have carrier proteins in the blood.
These have a high specificity and affinity for each steroid hormone, although not as high as their receptor proteins in the target tissue.
These binding protein in the blood belong to the albumin family of proteins.
It was once thought that the carrier proteins were necessary to keep the hydrophobic steroid hormones soluble in the blood.
While the steroids are highly insoluble, these hormones are soluble in the blood to a concentration of ~ 10-7M.
Their physiological working concentration is 10-9M to 10-12M.
Thus, solubility is not an issue at the very low physiological working concentration of the hormones.
It therefore seems unlikely that the steroid binding proteins have an important function holding the hydrophobic steroids in solution in the blood.
Another idea for the function of the carriers is that they provide a high-affinity site in the blood for the hormone to encourage the hormone to leave the hydrophobic environment inside the steroid producing cell.
In other words, they might aid in the release of the hormone.
Yet another possibility is that the carrier protects the steroid hormone from breakdown in the liver.
The liver is responsible for converting the steroid hormones to hydrophilic metabolites which can then be excreted from the body via the kidney (the kidney can only handle hydrophilic products).
Only the free, unbound form of the hormone can be taken up and metabolized by the liver.
It should be noted that only the free, unbound form of the hormone can be taken up by target tissue as well.
Thus, the steroid binding proteins could have some function in determining the level of free steroid available for target cell activation.
Finally, it should also be mentioned that only the free, unbound hormone can cross the blood-brain barrier to access brain targets.
Steroid-binding proteins could determine which steroid hormones can access the brain.