 Biochemical and cell physiological approaches have been more important than
molecular approaches in defining the role of regulatory domains within the nicotinic
receptor.
Biochemical and cell physiological approaches have been more important than
molecular approaches in defining the role of regulatory domains within the nicotinic
receptor. Biochemical and cell physiological approaches have been more important than
molecular approaches in defining the role of regulatory domains within the nicotinic
receptor.
Biochemical and cell physiological approaches have been more important than
molecular approaches in defining the role of regulatory domains within the nicotinic
receptor.
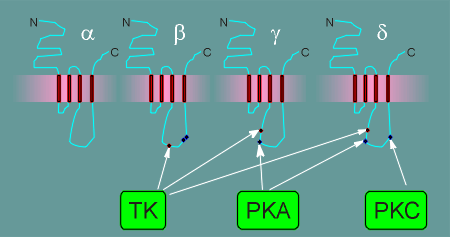
It has turned out that the nicotinic receptor is phosphorylated by at least three different kinases in the intracellular loops between transmembrane regions M3 and M4.
Two of these kinases are regulated by second messengers and are thus involved in cross-talk between the nicotinic receptors and G-protein coupled receptors.
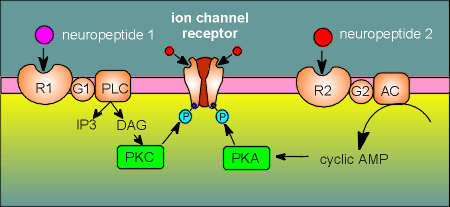 Protein kinase A (PKA) is regulated by the second messenger cyclic AMP, which
is generated by the enzyme adenylyl cyclase (AC).
Protein kinase A (PKA) is regulated by the second messenger cyclic AMP, which
is generated by the enzyme adenylyl cyclase (AC).
Protein kinase C (PKC) is regulated by the second messenger diacyl glycerol (DAG), which is generated by the enzyme phospolipase C (PLC).
(PLC also generates another second messenger, inositol triphosphate, IP3)
PKC phosphorylates the delta subunit of the nicotinic receptor while the PKA phosphorylates in both the gamma and delta subunit.
Through these phosphorylations neuropeptides, signaling through G protein coupled receptors, can influence the functioning of the nicotinic receptor.
In addition, the beta subunit has a phosphorylaton site for a tyrosine kinase (TK), a type of kinase that phosphorylates on the amino acid tyrosine (tyrosine kinases are the subject of one of the tutorials).
In general, the effect of phosphorylation of the nicotinic receptor is to desensitize it.
In the phosphorylated state the receptor closes despite the continued presence of acetylcholine.